Abstract
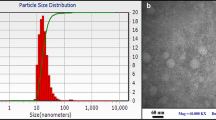
Cymbopogon citratus (DC) stapf. (Gramineae) is a herb known worldwide as lemongrass. The oil obtained, i.e., lemongrass oil has emerged as one among the most relevant natural oils in the pharmaceutical industry owing to its extensive pharmacological and therapeutic benefits including antioxidant, antimicrobial, antiviral and anticancer properties. However, its usage in novel formulations is constrained because of its instability and volatility. To address these concerns, the present study aims to formulate lemongrass-loaded SLN (LGSLN) using hot water titration technique. In the Smix, Tween 80 was selected as a surfactant component, while ethanol was taken as a co-surfactant. Different ratios of Smix (1:1, 1:2, 1:3, 2:1 and 3:1) were utilized to formulate LG-loaded SLN. The results indicated the fact that the LGSLN formulation (abbreviated as LGSLN1), containing lipid phase 10% w/w (i.e., LG 3.33% and SA 6.67%), Tween 80 (20% w/w), ethanol (20% w/w) and distilled water (50% w/w), revealed suitable nanometric size (142.3 ± 5.96 nm) with a high zeta potential value (− 29.12 ± 1.7 mV) and a high entrapment efficiency (77.02 ± 8.12%). A rapid drug release (71.65 ± 5.33%) was observed for LGSLN1 in a time span of 24 h. Additionally, the highest values for steady-state flux (Jss; 0.6133 ± 0.0361 mg/cm2/h), permeability coefficient (Kp; 0.4573 ± 0.0141 (cm/h) × 102) and enhancement ratio (Er; 13.50) was also conferred by LGSLN1. Based on in vitro study results, the developed SLN appeared as a potential carrier for enhanced topical administration of lemongrass oil. The observed results also indicated the fact that the phyto-cosmeceutical prospective of the nanolipidic carrier for topical administration of lemongrass oil utilizing pharmaceutically acceptable components can be explored further for widespread clinical applicability.



Similar content being viewed by others
Data availability
The authors confirm that the data supporting the study’s findings are included in the article.
References
Al-Harrasi A, Bhatia S, Behl T, Kaushik D (2022) Effects of essential oils on CNS. Role of essential oils in the management of COVID-19. CRC Press, Boca Raton, pp 269–297
Ali A, Ali A, Rahman MA, Warsi MH, Yusuf M, Alam P (2022) Development of nanogel loaded with lidocaine for wound-healing: illustration of improved drug deposition and skin safety analysis. Gels 8(8):466
Ascenso A, Ribeiro HM, Marques HC, Simoes S (2011) Topical delivery of antioxidants. Curr Drug Deliv 8:640–660
Bhaskar K, Anbu J, Ravichandiran V, Venkateswarlu V, Rao YM (2009) Lipid nanoparticles for transdermal delivery of flurbiprofen: formulation, in vitro, ex vivo and in vivo studies. Lipids Health Dis 8:6
Chen H, Zhong Q (2022) Physical and antimicrobial properties of self-emulsified nanoemulsions containing three synergistic essential oils. Int J Food Microbiol 365:109557
Cui HY, Wu J, Lin L (2016) Inhibitory effect of liposome-entrapped lemongrass oil on the growth of Listeria monocytogenes in cheese. Int J Dairy Sci 99(8):6097–6104
Dingler A, Gohla S (2002) Production of solid lipid nanoparticles (SLN): scaling up feasibilities. J Microencapsul 19:11–16
Dolatabadi JEN, Valizadeh H, Hamishehkar H (2015) Solid lipid nanoparticles as efficient drug and gene delivery systems: recent breakthroughs. Adv Pharm Bull 5:151–159
Faheem F, Liu ZW, Rabail R, Haq IU, Gul M, Bryła M, Roszko M, Kieliszek M, Din A, Aadil RM (2022) Uncovering the industrial potentials of Lemongrass essential oil as a food preservative: a review. Antioxidants 11:720
Faiyazuddin M, Ahmad N, Baboota S, Ali J, Ahmad S, Akhtar J (2010a) Chromatographic analysis of trans and cis-citral in lemongrass oil and in a topical phytonanocosmeceutical formulation, and validation of the method. J Planar Chromatogr 23:233–236
Faiyazuddin M, Akhtar N, Akhter J, Suri S, Shakeel F, Shafiq S, Mustafa G (2010b) Production, characterization, in vitro and ex vivo studies of babchi oil-encapsulated nanostructured solid lipid carriers produced by a hot aqueous titration method. Pharmazie 65:348–355
Fang JY, Fang CL, Liu CH, Su YH (2008) Lipid nanoparticles as vehicles for topical psoralen delivery: solid lipid nanoparticles (SLN) versus nanostructured lipid carriers (NLC). Eur J Pharm Biopharm 70(2):633–640
Freitas C, Müller RH (1999) Correlation between long-term stability of solid lipid nanoparticles (SLN™) and crystallinity of the lipid phase. Eur J Pharm Biopharm 47:125–132
Holland DB, Jeremy AH (2005) The role of inflammation in the pathogenesis of acne and acne scarring. Semin Cutan Med Surg 24:79–83
Irfan S, Zahra SM, Murtaza MA, Zainab S, Shafique B, Kanwal R, Roobab U, Ranjha MM (2022) Characterization of lemongrass oleoresins. Handbook of oleoresins. CRC Press, New York, pp 261–283
Jaber AA, Mirza MA, Anwer MK, Alshetaili AS, Alshahrani SM, Al-Shdefat RI, Talegaonkar S, Iqbal Z (2018) Mucoadhesive gels loaded with Ciclopirox olamine containing SLN for sustained vaginal drug delivery: in vitro and in vivo characterization. Lat Am J Pharm 37(2):388–400
Jenning V, Thünemann AF, Gohla SH (2000) Characterisation of a novel solid lipid nanoparticle carrier system based on binary mixtures of liquid and solid lipids. Int J Pharm 199:167–177
Jenning V, Lippacher A, Gohla SH (2002) Medium scale production of solid lipid nanoparticles (SLN) by high pressure homogenization. J Microencapsul 19:1–10
Kumar PS, Nattudurai G, Islam VI, Ignacimuthu S (2022) The effects of some essential oils on Alternaria alternata, a post-harvest phyto-pathogenic fungus in wheat by disrupting ergosterol biosynthesis. Phytoparasitica 50:513–525
Lertsatitthanakorn P, Taweechaisupapong S, Aromdee C, Khunkitti W (2006) In vitro bioactivities of essential oils used for acne control. Int J Aromather 16:43–49
Li P, Amit K, Wagner GA, RF, Krill S, Joshi YM, Serajuddin AT, (2005) Effect of combined use of non-ionic surfactants on formation of oil-in-water microemulsions. Int J Pharm 288:27–34
Liedtke S, Wissing S, Müller RH, Mäder K (2000) Influence of high pressure homogenisation equipment on nanodispersions characteristics. Int J Pharm 196:183–185
Ma JY, Hasham R, Abd Rasid ZI, Noor NM (2021) Formulation and characterization of nanostructured lipid carrier encapsulate lemongrass oil using ultrasonication technique. Chem Eng Trans 83:475–480
Masurkar SA, Chaudhari PR, Shidore VB, Kamble SP (2011) Rapid biosynthesis of silver nanoparticles using Cymbopogan citratus (lemongrass) and its antimicrobial activity. Nano-Micro Lett 3(3):189–194
Mirza MA, Ahmad S, Mallick MN, Manzoor N, Talegaonkar S, Iqbal Z (2013) Development of a novel synergistic thermosensitive gel for vaginal candidiasis: an in vitro, in vivo evaluation. Colloids Surf B Biointerfaces 103:275–282
Mukarram M, Choudhary S, Khan MA, Poltronieri P, Khan MMA, Ali J, Kurjak D, Shahid M (2021) Lemongrass essential oil components with antimicrobial and anticancer activities. Antioxidants (basel) 11(1):20
Muller RH, Lucks JS (1996) European Patent EP 0605497
Onawunmi GO, Yisak WA, Ogunlana EOJ (1984) Antibacterial constituents in the essential oil of Cymbopogon citratus (DC.) Stapf. Ethnopharmacol 12:279–283
Qadir A, Faiyazuddin MD, Hussain MT, Alshammari TM, Shakeel F (2016) Critical steps and energetics involved in a successful development of a stable nanoemulsion. J Mol Liq 214:7–18
Rauber CS, Guterres SS, Schapoval EES (2005) LC determination of citral in Cymbopogon citratus volatile oil. J Pharma Biomed Anal 37:597–601
Salvia-Trujillo L, Rojas-Graü MA, Soliva-Fortuny R, Martín-Belloso O (2014) Impact of microfluidization or ultrasound processing on the antimicrobial activity against Escherichia coli of lemongrass oil-loaded nanoemulsions. Food Control 37:292–297
Sinha P, Srivastava N, Rai VK, Mishra R, Ajayakumar PV, Yadav NP (2019) A novel approach for dermal controlled release of salicylic acid for improved anti-inflammatory action: combination of hydrophilic-lipophilic balance and response surface methodology. J Drug Deliv Sci Technol 52:870–884
Souza VVMA, Almeida JM, Barbosa LN, Silva NCC (2022) Citral, carvacrol, eugenol and thymol: antimicrobial activity and its application in food. J Essent Oil Res 34(3):181–194
Thatipamula RP, Palem CR, Gannu R, Mudragada S, Yamsani MR (2011) Formulation and in vitro characterization of domperidone loaded solid lipid nanoparticles and nanostructured lipid carriers. Daru J Faculty Pharm Tehran Univ Med Sci 19(1):23
Thomas L, Zakir F, Mirza MA, Anwer MK, Ahmad FJ, Iqbal Z (2017) Development of Curcumin loaded chitosan polymer based nanoemulsion gel: In vitro, ex vivo evaluation and in vivo wound healing studies. Int J Biol Macromol 101:569–579
Trotta M, Debernardi F, Caputo O (2003) Preparation of solid lipid nanoparticles by a solvent emulsification-diffusion technique. Int J Pharm 257:153–160
Valková V, Ďúranová H, Galovičová L, Borotová P, Vukovic NL, Vukic M, Kačániová M (2022) Cymbopogon citratus Essential Oil: its application as an antimicrobial agent in food preservation. Agronomy 12:155
Yadav V, Mahor A, Prajapati S, Alok S, Verma A, Kumar N, Kumar S (2014) Solid lipid nanoparticles (sln): formulation by high pressure homogenization. World J Pharmacy Pharm Sci 3:1200–1203
Yuan H, Huang LF, Du YZ, Ying XY, You J, Hu FQ, Zeng S (2008) Solid lipid nanoparticles prepared by solvent diffusion method in a nanoreactor system. Colloids Surf B Biointerfaces 61:132–137
Zakir F, Ahmad A, Mirza MA, Kohli K, Ahmad FJ (2021) Exploration of a transdermal nanoemulgel as an alternative therapy for postmenopausal osteoporosis. J Drug Deliv Sci Technol 65:102745
Zhao M, Zhang Y, Zou C, Dai C, Gao M, Li Y, Lv W, Jiang J, Wu Y (2017) Can more nanoparticles induce larger viscosities of nanoparticle-enhanced wormlike micellar system (NEWMS)? Materials 10(9):1096
Zouboulis C (2001) Exploration of retinoid activity and the role of inflammation in acne: issues affecting future directions for acne therapy. J Eur Acad Dermatol Venereol 15:63–67
Acknowledgements
Authors are thankful to the Taif University Researchers Supporting Project (Number TURSP-2020/124), Taif University, Taif, Saudi Arabia, for supporting this work.
Funding
This research was funded by Taif University Research Supporting Project number ‘TURSP-2020/124.’
Author information
Authors and Affiliations
Contributions
The authors confirm their contribution as follows: study conception and design: AbA, and AmA; data collection and analysis: MHW, WA, MA, and SAHA; interpretation of results: WA, AbA and AmA; draft manuscript preparation: MA, AbA, and SAHA; Funding acquisition: AbA.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The study was approved by Ethics Committee, Taif University, Saudi Arabia.
Supplementary Information
Below is the link to the electronic supplementary material.

Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ali, A., Ali, A., Warsi, M.H. et al. Formulation of lemongrass oil (Cymbopogon citratus)-loaded solid lipid nanoparticles: an in vitro assessment study. 3 Biotech 13, 318 (2023). https://doi.org/10.1007/s13205-023-03726-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-023-03726-5