Abstract
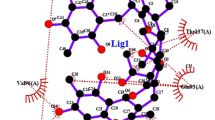
Typha domingensis Pers. is known for its medicinal properties. Although traditionally T. domingensis Pers. has been used for wound healing, yet scientific investigations reporting its ability to heal wounds are lacking. Phytochemical profiling of T. domingensis Pers. inflorescence crude extract was carried out by LC–MS analysis. Ten phytochemicals were selected for in silico analysis based on retention time, mass-to-charge ratio and resolution of mass spectrum. Molecular docking of all ten compounds was done against selected wound healing biomarkers viz., interleukin 6(IL-6), interleukin β (IL-β), insulin-like growth factor tyrosine kinase receptor (IGF-1R) and transformation growth factor β (TGF-β). Based on this, catechin, mesalazine and piperazine were subjected for in vitro cell migration assay (3T3 L1 mouse fibroblast cell line) to assess their wound healing potentials. Molecular docking revealed that mesalazine, catechin, and piperazine have potential ligands based on lowest docking energy (ranging from − 4.1587 to − 0.972), Glide E score (ranging from − 26.929 to − 57.882), Glide G score (ranging from − 4.16 to − 7.972) and numbers of hydrogen bonds compared to other compounds studied. The migration assay revealed that, compared to control (52.5%), T. domingensis Pers. inflorescence crude extract showed maximum wound healing potential (80%) followed by Catechin (66.8%) Mesalazine (58.3%) and Piperazine (51.2%). The combined in silico and in vitro approach opens new dimension for designing innovative therapeutics to manage different types of wounds.







Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this article.
References
Ali G, Ashraf S, El-Sayed A, Patel JS, Green KB, Ali M, Brennan M, Norman D (2016) Ex vivo application of secreted metabolites produced by soil- inhabiting Bacillus spp. efficiently controls foliar diseases caused by Alternaria spp. Appl Environ Microbiol 82:478–490
Alireza FA, Ghodsi S, Emami S, Najjari S, Samadi N, Faramarzi MA, Beikmohammadi L, Shirazi FH, Shafiee A (2006) Synthesis and antibacterial activity of new fluoroquinolones containing a substituted N-(phenethyl)piperazine moiety. Bioorg Med Chem Lett 16:3499
Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic Canic M (2008) Growth factors and cytokines in wound healing. Wound Repair and Regeneration 16:585–601
Bensky, D., Gamble, A., Kaptchuk, T., 1993.Chinese herbal medicine. In: Material Medica. Revised Edition. Eastland press, Seatle, 1993, pp. 70–71, 277–278.
Berkheij M, Van der Sluis L, Sewing C, Den Boer DJ, Terpstra JW, Hiemstra H, Iwema Bakker WI, Van Den Hoogenband A, Van Maarseveen JH (2005) Synthesis of 2-substituted piperazines via direct alpha-lithation. Tetrahedron Lett 46:2369–2371
Biswas TK, Mukherjee B (2003) Plant medicines of Indian origin for wound healing activity: A Review. Int J Low Extrem Wounds 2:25–39
Bokhari MH (1983) The aquatic plants of Iran and Pakistan. III Typhaceae Biologia 29:85–91
Borris RP (1996) Natural products research: perspectives from a major pharmaceutical company. J Ethnopharmacol 51:29–38
Chan MMY, Dunne F, Ho CT, Huang HI (1999) Inhibition of inducible nitric oxide synthase gene expression and enzyme activity by epigallocatechingallate, a natural product from green tea. Biochem Pharmacol 54:1281–1286
Chandra JN, Sadashiva CT, Kavitha CV, Rangappa KS (2006) Synthesis and in vitro antimicrobial studies of medicinally important novel N-alkyl and N- sulfonyl derivatives of 1-[bis(4-fluorophenyl)-methyl]piperazine. Bioorg Med Chem 14:6621
Chen M, Zheng H, Yin LP, Xie CG (2010) Is oral administration of Chinese herbal medicine effective and safe as an adjunctive therapy for managing diabetic foot ulcers? a systematic review and meta-analysis. J Altern Complement Med 16:889–898
Cook, C. D. K., 1980. Typhaceae. In Flora Europaea, Tutin, T. G. et al. (eds.) Vol-5 Cambridge University Press. 275–276.
Cory G (2011) Scratch wound assay. Methods Mol Biol 769:25–30
Crouvezier S, Powell B, Keir D, Yaqoob P (2000) The effects of phenolic components of tea on the production of Pro- and anti-inflammatory cytokines by human leukocytes In vitro Cytokine. Cytokine 13:280–286
Decaestecker C, Debeir O, Van Ham P, Kiss R (2007) Can anti-migratory drugs be screened in vitro? A review of 2D and 3D assays for the quantitative analysis of cell migration. Med Res Rev 27(2):149–176. https://doi.org/10.1002/med.20078, PMID 16888756
Desmoulière A, Geinoz A, Gabbiani F, Gabbiani G (1993) Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 122(1):103–111
Dewangan H, Bais M, Jaiswal V, Verma VK (2012) Potential wound healing activity of the ethanolic extract of Solanum xanthocarpum schrad and wend l leaves. Pak J Pharm Sci 25:189–194
Eming, S.A., Martin, P., Tomic-Canic, M., 2014.Wound repair and regeneration: mechanisms, signaling, and translation. Sci. Transl. Med. 6, (265), 265sr6.
Evrard SM, d’Audigier C, Mauge L, Israel-Biet D, Guerin CL, Bieche I, Kovacic JC, Fischer AM, Gaussem P, Smadja DM (2012) The profibrotic cytokine transforming growth factor-beta1 increases endothelial progenitor cell angiogenic properties. J Thromb Haemost 10(4):670. https://doi.org/10.1111/j.1538-7836.2012.04644.x
Finlayson M, Forrester R, Mitchell D, Chick A (1985) Identification of Native Typha species in Australia. Aust J Bot 33:101–107
Gailit J, Clark RA, Welch MP (1994) TGF-β1 stimulates expression of keratinocyte integrins during reepithelialization of cutaneous wounds. J Investig Dermatol 103(2):221–227
Gisbert JP, Gomollón F, Maté J, Pajares JM (2002) Role of 5-aminosalicylic acid (5-ASA) in treatment of inflammatory bowel disease: a systemic review. Dig Dis Sci 47:471–488
Govaerts, R., 2018 World Checklist of Typhaceae.Facilitated by the Royal Botanic Gardens, Kew. Published on the Internet; http://wcsp.science.kew.org/ (Accessed 10 February 2018).
Guedes IA, de Magalhães CS, Dardenne LE (2014) Receptor-ligand molecular docking. Biophys Rev 6:75–87
Guo S, Dipietro LA (2010) Factors affecting wound healing. J Dent Res 89:219–229
Halperin I, Ma B, Wolfson H, Nussinov R (2002) Principles of docking: an overview of search algorithms and a guide to scoring functions. PROTEINS: St Fun Gen 47(4):409–443
Hamdi SMM, Assadi M (2003) Typhaceae. In: SMM Hamdi M (ed) Flora of Iran 42 Verlag Paul Parey, Berlin and Hamburg, 299–317
Him-Che Y (1985) Hand book of Chinese Herbs and Formulas. Institute of Chinese Medicine, Los Angles, p 5
Hinz B (2007) Formation and function of the myofibroblast during tissue repair. J Investig Dermatol 127:526–537
Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G (2007) The myofibroblast: one function, multiple origins. Am J Pathol 170(6):1807–1816
Horwitz R, Webb D (2003) Cell migration.Current Biology, 13, R756–R759.
Jacinto A, Martinez-Arias P, Martin (2001) Mechanisms of epithelial fusion and repair, Nat. Cell Biol. 3 E117–E123
Jung-Jin L, Hyoseok Yi, In-Su K, Yohan K, Nguyen XN, Young HK, Myung C-S (2012) (2S) Naringenin from Typha angustata inhibits vascular smooth muscle cell proliferation via a G0/G1 arrest. J Ethnopharmacol 139(3):873–878. https://doi.org/10.1016/j.jep.2011.12.038
Kalirajan R, Vivek K, Sankar S, Jubie S (2012) Docking studies, synthesis, characterization of some novel oxazine substituted 9-anilinoacridine derivatives and evaluation for their anti oxidant and anticancer activities as topo isomerase II inhibitors. Eur J Med Chem 56:217–224. https://doi.org/10.1016/j.ejmech.2012.08.025. PMid:22982526
Kamboj VP (2000) Herbal medicine.Curr Sci 78, 35–51.
Kolhe VN, Pawar CR, Khedkar PA (2011) Anti- inflammatory activity of leaves of Typha angustata (Typhaceae). Int. J. Res. Ayurveda Pharm. 2, 1598-1600
Komakech R, Matsabisa MG, Kang Y (2019) The wound healing potential of Aspilia Africana (Pers.) C.D Adams (asteraceae). Evid Based Complement Alternat Med 2019:1–12
Kramer N, Walzl A, Unger C, Rosner M, Krupitza G, Hengstschläger M, Dolznig H (2013) In vitro cell migration and invasion assays. Mutat Res 752(1):10–24
Kuehn MM, White BN (1999) Morphological analysis of genetically identified cattails Typha latifolia, Typha angustifolia, and Typha glauca. Can J Bot 77:906–912
Lengauer T, Rarey M (1996) Computational methods for biomolecular docking. Curr Opin Struct Biol 6:402–406
Liang CC, Park AY, Guan JL (2007) In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Proto 2(2):329–333
Lokesh BV, Prasad YR, Shaik AB (2019) Synthesis, Biological evaluation and molecular docking studies of new pyrazolines as an antitubercular and cytotoxic agents. Infectious Disorders-Drug Targets 19(3):310–321
Moss AC, Peppercorn MA (2007) The risks and the benefits of mesalazine as a treatment for ulcerative colitis. Expert Opin Drug Saf 6:99–107
Mustoe TA, O’Shaughnessy K, Kloeters O (2006) Chronic wound pathogenesis and current treatment strategies: a unifying hypothesis. Plast Reconstr Surg 117(7 Suppl):35S-41S
Nascimento JE et al (2018) Chemical composition and antifungal in vitro and insilico, antioxidant and anticholinesterase activities of extracts and constituents of Ouratea fieldingiana (DC.) Baill. J Evid Based Complementary Altern Med 1–13
Ngo LT, Okogun JI, Folk WR (2013) 21st Century natural product research and drug development and traditional medicines. Nat Prod Rep 30(4):584–592
Nostro, A., Germano, M.P., D’ Angelo, A., Marino.A., Cannatelli. M.A., 2000. Extraction methods and bioautography for evaluation of medicinal plant antimicrobial activity. Lett. Appl. Microbiol. 30, 379-385
Oh-oka K, Kojima Y, Uchida K, Yoda K, Ishimaru K, Nakajima S et al (2017) Induction of Colonic Regulatory T Cells by Mesalamine by Activating the Aryl Hydrocarbon Receptor. Cell Mol Gastroenterol Hepatol 4:135–151
Petrie RJ, Doyle AD, Yamada KM (2009) Random versus directionally persistent cell migration Nat. Rev Cell Bio 10:538–549
Pirbalouti GA, Koohpayeh A, Karimi I (2010) The wound healing activity of flower extract of Punica granatum and Achilleakellalensis in wister rats. Acta Pol Pharm 67:1070–1110
Reinke JM, Sorg H (2012) Wound repair and regeneration. Eur Surg Res 49(1):35–43
Rohs R, Bloch I, Sklenar H, Shakked Z (2005) Molecular flexibility in ab-initio drug docking to DNA: binding-site and binding-mode transitions in all-atom Monte Carlo simulations. Nucl Acids Res 33:048–7057
Saha S (1968) The Genus Typha in India–its distribution and uses. J Botanical Socety Bengal 22:11–18
Schultz T (2003) Quantitative structure-activity relationships (QSARs) in toxicology: a historical perspective. J Mol Struct (thoechem) 622:1–22
Schultz GS, Wysocki A (2009) Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regeneration 17:153–162
Seeliger D, de Groot BL (2010) Ligand docking and binding site analysis with PyMOL and AutodockVina. J Comput Aided Mol Des 24:417–422
Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT (2009) Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regeneration 17:763–771
Siavash M, Shokri S, Haghighi S, Shahtalebi MA, Farajzadehgan Z (2015) The efficacy of topical royal jelly on healing of diabetic foot ulcers: a double-blind placebo controlled clinical trial. Int Wound J 12(2):137–142
Simpson KJ, Selfors LM, Bui J, Reynolds A, Leake D, Khvorova A, Brugge JS (2008) Identification of genes that regulate epithelial cell migration using an siRNA screening approach. Nat Cell Biol 10(9):1027–1038
Stephen YG, Emelia K, Francis A, Kofi A, Eric W (2010) Wound healing properties and kill kinetics of Clerodendron splendens G Don, A Ghanaian wound healing plant. Pharmacognosy Res 2:63–68
Strbo N, Yin N, Stojadinovic O (2014) Innate and Adaptive Immune Responses in Wound Epithelialization. Adv Wound Care (new Rochelle) 3:492–501
Subhashini S, Arunachalam KD (2011) Investigations on the phytochemical activities and wound healing properties of Adhatoda vasica leave in Swiss albino mice. Afr J Plant Sci 5:133–145
Sun K, Simpson D (2010) Typhaceae. In: Wu ZY, Raven PH, Hong DY (eds) Flora of China. Science Press and Missouri Botanical Garden Press, pp 158–163
Sun BK, Siprashvili Z, Khavari PA (2014) Advances in skin grafting and treatment of cutaneous wounds. Science 346:941–945
Walter MNM, Wright KT, Fuller HR, MacNeil SM, Johnson WEB (2010) Mesenchymal stem cell conditioned medium accelerates skin wound healing: an in vitro study of fibroblast and keratinocyte scratch assays. Exp Cell Res 316(7):1271–1281. https://doi.org/10.1016/j.yexcr.2010.02.026. PMID20206158
Werner S, Antsiferova M (2016) Wound healing: an orchestrated process of cell cycle, adhesion and signaling. In Encylopedia of cell biology (Elsevier), 216–222.
Yang F, de Villiers WJ, McClain CJ, Varilek GW (1998) Green tea polyphenols block endotoxin-induced tumor necrosis factor-production and lethality in a murine model. J Nutraceuticals 128:2334–2340
Yen GC, Chen HY (1998) Scavenging effect of various tea extracts on superoxide derived from the metabolism of mutagens. Biosci Biotechnol Biochem 62(9):1768–1770
Ziraldo C, Mi Q, An G, Vodovotz Y (2013) Computational modeling of inflammation and wound healing. Adv Wound Care (new Rochelle) 2(9):527–537
Acknowledgements
The authors acknowledge management of Uka Tarsadia University in providing the necessary facility to carry out the work. The authors are also thankful to Sophisticated Analytical Instrument Facility (SAIF), IIT Bombay, for LC–MS analysis and Cellkraft Pvt. Ltd, Bangluru, India for Cell migration assay.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or not for profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Rights and permissions
About this article
Cite this article
Saha, S., Naik, J., Amaresan, N. et al. In silico analysis of Typha domingensis Pers. phytocompounds against wound healing biomarkers and ascertaining through in vitro cell migration assay. 3 Biotech 12, 166 (2022). https://doi.org/10.1007/s13205-022-03229-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-022-03229-9