Abstract
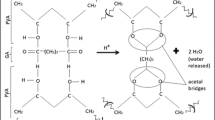
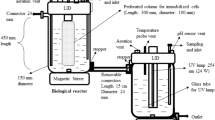
This work investigated the properties of Paracoccus yeei VKM B-3302 bacteria isolated from activated sludge and immobilized in an N-vinylpyrrolidone-modified poly(vinyl alcohol) matrix. The developed hydrogel formed a network structure to enable the entrapment of microbial cells with their viability and biocatalytic properties preserved, which ensured the technological possibility of replicating expendable biosensor receptor elements. A new ratio of the components for the synthesis selected in this work enabled producing a copolymer of an earlier undescribed chemical structure, which can be efficiently used for immobilization of highly sensitive P. yeei bacteria. A biological oxygen demand (BOD) biosensor with these bacteria and matrix was shown to possess a long-time stability exceeding that described earlier, to have a broad substrate specificity and to exceed approximately tenfold the nearest analogues by its sensitivity and the lower boundary value of 0.05 mg/dm3. The biosensor enabled assays of water samples initially attributed to pure samples (the BOD range, 0.05–5.0 mg/dm3). BOD assays of water samples from various sources showed the use of the receptor element of this composition to enable the data that closely correlated with the standard method (R2 = 0.9990).







Similar content being viewed by others
References
Abrevaya XC, Sacco NJ, Bonetto MC, Hilding-Ohlsson A, Cortón E (2014) Analytical applications of microbial fuel cells Part I: Biochemical oxygen demand. Biosensors Bioelectron 63:580–590
Aleshina EY, Yudanova TN, Skokova IF (2001) Production and properties of polyvinyl alcohol spinning solutions containing protease C and polyhexamethylene guanidine. Fibre Chem 33(6):421–423
Arlyapov VA, Yudina NYu, Asulyan LD, Alferov SV, Alferov VA, Reshetilov AN (2013) BOD biosensor based on the yeast Debaryomyces hansenii immobilized in poly(vinyl alcohol) modified by N-vinylpyrrolidone. Enzyme Microb Technol 53:257–262
Bazilyuk TN, Melnik NP, Menzheres GYa (2003) Modification of poly(vinyl alcohol) by poly-N-vinylpyrrolidone. Voprosy Khimii I Khimicheskoi Tekhnologii 1:57–60 (in Russian)
Buenger D, Topuz F, Groll J (2012) Hydrogels in sensing applications. Prog Polym Sci 37(12):1678–1719
Burlage RS, Tillmann J (2017) Biosensors of bacterial cells. J Microbiol Methods 138:2–11
Commault A, Weld RJ (2016) Whole-cell biosensors for monitoring bioremediation. In: Lear G (ed) Biofilms in bioremediation: current research and emerging technologies, chap 5. Caister Academic Press, pp 75–92
Demakov VA, Maksimova YuG, Maksimov AYu (2008) Immobilization of microbial cells: biotechnological aspects. Biotekhnologiia 2:30–45 (in Russian)
Fang D et al (2016) A reagentless electrochemical biosensor based on thionine wrapped E. coli and chitosan-entrapped carbon nanodots film modified glassy carbon electrode for wastewater toxicity assessment. Electrochimica Acta 222:303–311
Federal Environmental Regulatory Documents (PND F) 14. 1:2:3:4. 123–97 (1997) Quantitative chemical analysis of waters. Methods of measuring the biochemical oxygen demand after n days of incubation (BODtotal) in surface fresh, ground, drinking, effluent and purified effluent waters. Moscow, 25 p. (in Russian).
Fine T, Leskinen P, Isobe T, Shiraishi H, Morita M, Marks RS, Virta M (2006) Luminescent yeast cells entrapped in hydrogels for estrogenic endocrine disrupting chemical biodetection. Biosens Bioelectron 21(12):2263–2269
Guisan JM editor (2006) Immobilization of enzymes and cells. 2nd ed. Totowa, New Jersey: Humana Press 450 p
ISO 5815–1:2003, 2003. Water Quality – Determination of Biochemical Oxygen Demand after n Days (BODn), Part 1: Dilution and Seeding Method with Allylthiourea Addition
Jouanneau S, Recoules L, Durand MJ, Boukabache A, Picot V, Primault Y, Thouand G (2014) Methods for assessing biochemical oxygen demand (BOD): A review. Water Res 49:62–82
Kharkova AS, Arlyapov VA, Turovskaya AD, Avtukh AN, Starodumova IP, Reshetilov AN (2019) Mediator BOD biosensor based on cells of microorganisms isolated from activated sludge. Appl Biochem Microbiol 55(2):189–197
Li Y, Sun J, Wang J, Bian C, Tong J, Li Y, Xia S (2016) A single-layer structured microbial sensor for fast detection of biochemical oxygen demand. Biochem Eng J 112:219–225
Martínez M, Hilding-Ohlsson A, Viale AA, Cortón E (2007) Membrane entrapped Saccharomyces cerevisiae in a biosensor-like device as a generic rapid method to study cellular metabolism. J Biochem Biophys Methods 70(3):455–464
Ponamoreva ON, Arlyapov VA, Alferov VA, Reshetilov AN (2011) Microbial biosensors for detection of biological oxygen demand (a review). Appl Biochem Microbiol 47(1):1–11
Raud M, Tenno T, Jogi E, Kikas T (2012) Comparative study of semi-specific Aeromonas hydrophila and universal Pseudomonas fluorescens biosensors for BOD measurements in meat industry wastewaters. Enzyme Microb Technol 50(4–5):221–226
Szczesna-Antczak M, Antczak T, Bielecki S (2004) Stability of extracellular proteinase productivity by Bacillus subtilis cells immobilized in PVA-cryogel. Enzyme Microb Technol 34(2):168–176
Tag K, Lehmann M, Chan C, Renneberg R, Riedel K, Kunze G (2000) Measurement of biodegradable substances with a mycelia-sensor based on the salt tolerant yeast Arxula adeninivorans LS3. Sensors and Actuators B 67:142–148
Wang J, Bian C, Li Y, Tong J, Sun J, Hong W, Xia S (2015) A renewable BOD microsensor based on magnetically functionalized microorganism and ultramicroelectrode array. IEEE Sensors 2015:1–4
Yudina NYu, Arlyapov VA, Chepurnova MA, Alferov SV, Reshetilov AN (2015) A yeast co-culture-based biosensor for determination of waste water contamination levels. Enzyme Microb Technol 78:46–53
Zhanybekova AG et al (2013) N-Vinylpyrrolidone as a source of new excipients for pharmaceutical technology. Vestnik Kazakhskogo Natsionalnogo Meditsynskogo Universiteta 5–3:101–103 (in Russian)
Acknowledgements
The reported study was funded by the RFBR, project number 20–33-70078, and a grant of the President of the Russian Federation for the State Support of Young Russian PhD Scientists, agreement No. MK-1349.2020.3. The authors acknowledge Victor Selivanov for providing linguistic help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arlyapov, V.A., Yudina, N.Y., Asulyan, L.D. et al. Registration of BOD using Paracoccus yeei bacteria isolated from activated sludge. 3 Biotech 10, 207 (2020). https://doi.org/10.1007/s13205-020-02199-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-020-02199-0