Abstract
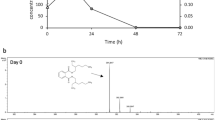
The present study was intended to investigate the biodegradation of acephate in aqueous media in the presence and in the absence of metal ions [Fe(III) and Cu(II)], and humic acid (HA). Biodegradations were performed using Pseudomonas pseudoalcaligenes PS-5 (PS-5) isolated from the heavy metal polluted site. Biodegradations were monitored by UV–Visible, FTIR, and electron spray ionization–mass spectrometry (ESI–MS) analyses. ESI–MS analysis revealed that PS-5 degraded acephate to two metabolites showing intense ions at mass-to-charge ratios (m/z) 62 and 97. The observed kinetic was the pseudo-first order, and half-life periods (t 1/2) were 2.79 d−1 (of PS-5 + acephate), 3.45 d−1 [of PS-5 + acephate + Fe(III)], 3.16 d−1 [of PS-5 + acephate + Cu(II)], and 5.54 d−1 (of PS-5 + acephate + HA). A significant decrease in degradation rate of acephate was noticed in the presence of HA, and the same was confirmed by UV–Visible and TGA analyses. Strong aggregation behavior of acephate with humic acid in aqueous media was the major cause behind the slow degradation rate of acephate . New results on acephate metabolism by strain PS-5 in the presence and in the absence of metal ions [Fe(III) and Cu(II)] and humic acid were obtained. Results confirmed that Pseudomonas pseudoalcaligenes strain PS-5 was capable of mineralization of the acephate without formation of toxic metabolite methamidophos. More significantly, the Pseudomonas pseudoalcaligenes strain PS-5 could be useful as potential biological agents in effective bioremediation campaign for multi-polluted environments.






Similar content being viewed by others
References
Albers CN, Banta GT, Hansen PE, Jacobsen OS (2009) The influence of organic matter on sorption and fate of glyphosate in soil—comparing different soils and humic substances. Environ Pollut 157:2865–2870. doi:10.1016/j.envpol.2009.04.004
Arias-Estevez M, Astray G, Cid A, Fernández-Gándara D, García-Río L, Mejuto JC (2008) Influence of colloid suspensions of humic acids upon the alkaline fading of carbocations. J Phys Org Chem 21:555–560. doi:10.1002/poc.1317
Astray G, García Río L, Lodeiro C, Mejuto JC, Moldes O, Morales J, Moyano F (2010) Influence of colloid suspensions of humic acids on the alkaline hydrolysis of N-methyl-N-nitroso-p-toluene sulfonamide. Int J Chem Kinet 42:316–322. doi:10.1002/kin.20481
Brigante M, Zaninia G, Avena M (2009) Effect of pHanions and cations on the dissolution kinetics of humic acid particles. Colloids Surf A 347:180–186. doi:10.1016/j.colsurfa.2009.04.003
Chai LK, Wong MH, Mohd-Tahir N, Hansen HCB (2010) Degradation and mineralization kinetics of acephate in humid tropic soils of Malaysia. Chemosphere 79:434–440. doi:10.1016/j.chemosphere.2010.01.046
Charlesworth S, Everett M, Mccarthy R (2003) A comparative study of heavy metal concentration and distribution in deposited street dusts in a large and a small urban area: Birmingham and Coventry West Midlands UK. Environ Int 29:563–573. doi:10.1016/S0160-4120(03)00015-1
Kim JY, Myung JH, Ahn JS (1998) Heavy metal speciation in dusts and stream sediments in the Taejon area Korea. J Geochem Explor 64:409–419. doi:10.3390/ijerph13080820
Kumar V, Upadhyay N, Wasit AB, Singh S, Kaur P (2013) spectroscopic methods for the detection of organophosphate pesticides—a preview. Curr World Environ 8:313–319. doi:10.12944/CWE.8.2.19
Kumar V, Upadhyay N, Kumar V, Kaur S, Singh J, Singh S, Datta S (2014) Environmental exposure and health risks of the insecticide monocrotophos—a Review. J Bio Env Sci 5:111–120
Kumar V, Upadhyay N, Kumar V, Sharma S (2015a) A review on sample preparation and chromatographic determination of acephate and methamidophos in different samples. Arab J Chem 8:624–631. doi:10.1016/j.arabjc.2014.12.007
Kumar V, Singh S, Singh J, Upadhyay N (2015b) Potential of plant growth promoting traits by bacteria isolated from heavy metal contaminated soils. Bull Environ Contam Toxicol 94:807–815. doi:10.1007/s00128-015-1523-7
Kumar V, Upadhyay N, Manhas A (2015c) Designingsynthesescharacterizationcomputational study and biological activities of silver-phenothiazine metal complex. J Mol Str 1099:135–140. doi:10.1016/j.molstruc.2015.06.055
Kumar V, Kumar V, Upadhyay N, Sharma S (2015d) Interactions of atrazine with transition metal ions in aqueous media: experimental and computational approach. Biotech 5:791–798. doi:10.1007/s13205-015-0281-x
Kumar V, Kumar V, Kaur S, Singh S, Upadhyay N (2016) Unexpected formation of N-phenyl-thiophosphorohydrazidic acid O, S-dimethyl ester from acephate: chemical biotechnical and computational study. 3. Biotech 6:1–11. doi:10.1007/s13205-015-0313-6
Lakanen E, Ervio R (1971) A comparison of eight extractants for the determination of plant available micronutrients on soil. Acta Agralia Fennica 123:223–232
Leharne S, Charlesworth D, Choudhry B (1992) A survey of metal levels in street dusts in an inner London Neighbourhood. Environ Int 18:263–270. doi:10.1016/0160-4120(92)90109-H
Manzanilla-Cano JA, Barceló-Quintal MH, Reyes-Salas EO (2004) Electrochemical monitoring of methylparathion degradation in an acid aqueous medium in presence of Cu(II). J Environ Sci Health B 39:577–588. doi:10.1081/PFC-200026805
Manzanilla-Cano JA, Barceló-Quintal MH, Rendón-Osorio RB, Flores-Rodríguez J (2007) Effect of Fe(III) on acid degradation of methylparathion. J Environ Sci Health B 425:15–22. doi:10.1080/03601230701391740
Mazzei P, Piccolo A (2012) Quantitative evaluation of noncovalent interactions between glyphosate and dissolved humic substances by NMR spectroscopy. Environ Sci Technol 146:5939–5946. doi:10.1021/es300265a
Mazzeia P, Oschkinatb H, Piccolo A (2013) Reduced activity of alkaline phosphatase due to host–guest interactions with humic superstructures. Chemosphere 93:1972–1979. doi:10.1016/j.chemosphere.2013.07.015
Piccolo A, Celano G (1994) Hydrogen bonding interactions of the herbicide glyphosate with water soluble humic substances. Environ Toxicol Chem 13:1737–1741. doi:10.1002/etc.5620131104
Pinjari B, Novikov B, RezenomYH Russell DH, Wales ME, Siddavattam D (2012) Mineralization of acephate a recalcitrant organophosphate insecticide is initiated by a pseudomonad in environmental samples. PLoS ONE 7:31963–31970. doi:10.1371/journal.pone.0031963
Prasad R, Upadhyay N, Kumar V (2013) Simultaneous determination of seven carbamate pesticide residues in gram wheat lentil soybean fenugreek leaves and apple matrices. Microchem J 111:91–97. doi:10.1016/j.microc.2012.12.014
Ramu S, Seetharamana B (2014) Biodegradation of acephate and methamidophos by a soil bacterium Pseudomonas aeruginosastrain Is-6. J Environ Sci Health B 49:23–34. doi:10.1080/03601234.2013.836868
Sarkouhi M, Shamsipur M, Hassan J (2012) 31P-NMR evaluation of organophosphorus pesticides degradation through metal ion promoted hydrolysis. Environ Monit Assess 184:7383–7393. doi:10.1007/s10661-011-2507-7
Sarkouhi M, Shamsipur M, Hassan J (2016) Metal ion promoted degradation mechanism of chlorpyrifos and phoxim. Arab J Chem 9:43–47. doi:10.1016/j.arabjc.2012.04.026
Singh S, Singh N, Kumar V, Datta S, Wani AB, Singh D, Singh K, Singh J (2016) Toxicity monitoring and biodegradation of the fungicide carbendazim. Environ Chem Lett 14(3):317–329. doi:10.1007/s10311-016-0566-2
Smejkalova D, Piccolo A (2008) Host-guest interactions between 2,4-dichlorophenol and humic substances as evaluated by 1H NMR relaxation and diffusion ordered spectroscopy. Environ Sci Technol 42:8440–8445. doi:10.1021/es801809v
Smejkalova D, Spaccini R, Fontaine B, Piccolo A (2009) Binding of phenol and differently halogenated phenols to dissolved humic matter as measured by NMR spectroscopy. Environ Sci Technol 43:5377–5382. doi:10.1021/es900559b
Wang L, Wen Y, Guo X, Wang G, Li S, Jiang J (2010) Degradation of methamidophos by Hyphomicrobium species MAP-1 and the biochemical degradation pathway. Biodegradation 21:513–523. doi:10.1007/s10532-009-9320-9
Wei B, Yang L (2010) A review of heavy metal contaminations in urban soils urban road dusts and agricultural soils from China. Microchem J 94:99–107. doi:10.1016/j.microc.2009.09.014
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh, S., Kumar, V., Upadhyay, N. et al. Efficient biodegradation of acephate by Pseudomonas pseudoalcaligenes PS-5 in the presence and absence of heavy metal ions [Cu(II) and Fe(III)], and humic acid. 3 Biotech 7, 262 (2017). https://doi.org/10.1007/s13205-017-0900-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-017-0900-9