Abstract
Pollution due to the presence of nickel is of great importance because of its toxic nature and non-biodegradability. Nickel is one of the most common heavy metals used in the industry. The purpose of the study is to investigate the efficiency of modified multi-walled carbon nanotubes (MWCNTs) on the adsorptive removal of nickel (II) from its aqueous environment and also to survey the applicability of the linear and nonlinear adsorption kinetic models on the process. The effects of process parameters including pH (4, 5, 7, and 10), MWCNT dosage (2.5, 5, 10, and 12 mg/L), initial nickel (II) ion concentration (25, 50, 100, and 125 mg/L) and contact time (5, 10, 25, and 40 min) on the removal efficiency of nickel using MWCNTs were studied, and their optimum conditions were also determined. Maximum nickel removal efficiency of 82% was observed at the optimum conditions of pH 10, MWCNT dosage of 10mg/L, initial nickel concentration of 100 mg/L and contact time of 10 min. The experimental data fitted best into the linear Ho (I) kinetic models than the other linear models. The rate-determining step of the process was found to be chemisorption. The correlation coefficient R2 values obtained for the linear and nonlinear models revealed that the nonlinear Ho adsorption kinetic provided a better explanation of the adsorption kinetic data of nickel removal by MWCNTs.
Similar content being viewed by others
Introduction
Heavy metals are persistent in the environment and have led to many problems (Zhang et al. 2010). They enter into the aquatic ecosystems and can accumulate in tissues and organs of aquatic organisms which are being eventually introduced into the food chain (Agarwal et al. 2016). Nickel is one of the most common heavy metals; it is applied in many industrial processes including steel manufacturing and electroplating industries (Zhang et al. 2010; Agarwal et al. 2016; Aregawi and Mengistie 2013). Low quantities of nickel are required to make red cells in the human body. However, nickel is toxic when present at high concentrations. Nickel is usually introduced into the environment through industrial wastes which do not cause problems in the short term but can, in the long run, decrease body weight, and cause damage to the heart and liver (Agarwal et al. 2016; Samadi et al. 2013). The World Health Organization (WHO) has announced that the standard permissible level of nickel in drinking water should be less than 0.02 mg/L (Ajmal et al. 2000). In most cases, nickel can be found in industrial wastes, battery factories, silver refineries, and even leachate from landfill sites (Zhang et al. 2010). Its half-life is about 11 h. The most toxic form of nickel that is often found in factories is carbonic compounds (Samadi et al. 2013). Therefore, its reduction in industrial wastewater is very necessary.
There are several methods for removing heavy metals including methods of combinatorial neutralization, adsorption, and reduction by ultrasonic waves (Ajmal et al. 2000; Agarwal et al. 2016). Recently, the application of nanotechnology in the purification of pollutants has been further developed in light of the ease and cost-effectiveness of their use compared to the conventional physical methods (Kashitarash Esfahani et al. 2012; Stafiej and Pyrzynska 2008). Although the nanotubes porosity is remarkably small, nanotube membranes have shown that due to the smooth interior surface of the nanotubes, the flow rate is more than or equal to the ones that possess larger porosity (Stafiej and Pyrzynska 2008). Nanotubes are hollow circular structures made up of carbon atoms which can be arranged in single-wall or multi-wall formations and have metal-like conductivity properties (Agarwal et al. 2016; Samadi et al. 2013). The multi-walled carbon nanotubes (MWCNTs) have a very high surface area, high permeability, good thermal and mechanical stability (Agarwal et al. 2016). Different nano-materials have been used to remove all types of pollutants from the water, but the MWCNTs have been utilized due to their unique characteristics including high specific surface, small size, simplicity of the process of producing these nanotubes, and health and environmental importance (Stafiej and Pyrzynska 2008). These materials are durable and resistant to heat, and cleaning and reusing them is easy. The MWCNTs membranes can remove almost all types of water contaminants; these contaminations include bacteria, organic compounds, and turbidity (Li et al. 2007; Heidari et al. 2016).
The aim of this study is to investigate the applicability of multi-walled carbon nanotubes on the removal of nickel(II) ions from its aqueous solution and also, to investigate the effects of different environmental factors such as pH, MWCNTs dosage (mg/L), initial concentration of nickel (mg/L), and time of treatment (min) on the adsorption process. Finally, the kinetic (linear and nonlinear) adsorption models were applied to study the mechanism of the adsorption process and the rate determining step.
Materials and methods
Materials
The MWCNTs were purchased from the Oil Industry Research Institute, Iran. The MWCNTs have been produced by chemical vapor deposition (CVD), modified with H2O2. Nickel chloride salt (NiCl2∙4H2O, 99.9% purity) was used to make a standard Ni(II) stock solution. 1 N hydrochloric acid (HCl) and sodium hydroxide (NaOH) were used to adjust the pH of the solutions to the desired pH. All chemicals were purchased from Merck (Germany). All materials were used without further purification.
Characterization of the adsorbent
The specific surface of the MWCNTs was measured using the Brunauer–Emmett–Teller method (BET) (Gemini 2357, Micrometrics Instrument Corporation, USA). In order to determine the exact diameter of MWCNTs, some information about the morphology of absorbent surface was provided by means of a scanning electron microscopy, SEM (HITACHI Model S-3000H). The physical specifications of the MWCNTs are presented in Table 1.
Batch adsorption experiments
A stock solution of Ni(II) ion was prepared in accordance with the standard methods (APHA and WPCF 2005). A stock solution of the nickel (with a concentration of 1000 mg/L) was prepared by dissolving 4.06 g of nickel salt (NiCl2·6H2O)] in 1 L of double-distilled water. The prepared solutions were stored at 4°C until usage. Subsequently, other required working concentrations (25, 50, 100, and 125 mg/L) were prepared from the stock solution by dilution with double-distilled water. The effects of pH (4, 5, 7, and 10), MWCNTs dose (2.5, 5, 10, and 12 mg/L), contact time (5, 10, 25, 40 min), and initial nickel concentration (25, 50, 100, and 125 mg/L) on the adsorption of Ni(II) using MWCNTs were investigated. All adsorption experiments were performed at room temperature. A 100 mL solution of known concentration was poured into 250-mL Erlenmeyer flask, the pH was adjusted to the desired pH by drop-wise addition, and a known dosage of adsorbent was added. The flask and its contents were placed in a shaker of 180 rpm and mixed till the desired time of treatment. Then, the mixture was filtered using a filter paper (Whatman No. 40) to remove the adsorbent. The filtrate was tested for the residual nickel using an atomic absorption spectroscopy (AAS) machine (Shelton, USA) using the flame absorption method at 232 nm. The removal efficiency, %R and the sorption capacity of MWCNTs, qe under the studied parameters were calculated based on the following formulas (Ahmadi and Kord Mostafapour 2017a, b, c, d, Ahmadi et al. 2017, 2018; Rahdar et al. 2018a):
where C0 and Cf are the initial and final concentrations (mg/L). qe is the adsorption capacity (mg/g). Ce is the equilibrium concentration of nickel (mg/L). M is the weight of adsorbent (g), and V is the volume of the solution (L).
Results and discussion
The specific surface area is one of the parameters determining the adsorbing capability of an adsorbent substance. When the specific surface area of an adsorbent substance is high, there will be higher level of contact between the adsorbent and adsorbate (Igwegbe et al. 2018; Banerjee and Chattopadhyaya 2017). The BET surface area of MWCNTs was obtained as 270 m2/g. In general, the adsorption capability of the MWCNTs is credited to their rich surface area (Abdel-Ghani et al. 2015). The SEM of MWCNTs showed that the external diameter of MWCNTs is in 10–30 nm (nanoscale) range. The adsorbent, MWCNTs possess cylindrical shapes, curved, elongated, and tangled in nature (Abdel-Ghani et al. 2015; Sheng et al. 2010; Salam et al. 2011).
The effect of initial pH
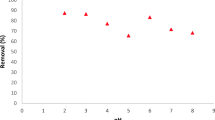
The effect of pH (4, 5, 7 and 10) on the removal of nickel (Ni(II)) on MWCNTs was studied at a contact time of 5 min, MWCNTs concentration of 5 mg/L, and initial Ni(II) ion concentration of 50 mg/L (Fig. 1). The removal efficiency of Ni(II) ions increased from 5 to 71% by increasing the pH from 4 to 10, and the amount of Ni(II) adsorbed on MWCNTs, qe also increased from 1 to 14.2 mg/g. The removal of nickel was not favorable at the acidic pH range but at the alkaline pH. Therefore, the optimal pH for Ni(II) ions adsorption was 10. This is due to the fact that the surface of the MWCNTs is negatively charged at alkali pH and the Ni(II) ions are positively charged; thus, electrostatic attraction is increased between them which led to the increase in the removal efficiency. This result is consistent with other studies (Zhang et al. 2010; Samadi et al. 2013; Stafiej and Pyrzynska 2008).
The effect of adsorbent dosage
Figure 2 shows the effect of MWCNTs dosage (2.5, 5, 10, and 12 mg/L) on the removal of Ni(II) at the optimum pH of 10, contact time of 5 min, and initial Ni(II) concentration of 50 mg/L. As the MWCNTs dosage was increased from 2.5 to 10 mg/L, the removal efficiency increased from 70 to 82.5%. On the other hand, increasing the adsorbent concentration from 2.5 to 12 mg/L decreased the amount of Ni(II) ions adsorbed on MWCNTs from 14 to 3.2 mg/g. The rate of removal significantly depends on the number of adsorption active sites available and by increasing the dosage of MWCNT to an appropriate level (Zhang et al. 2017; Samadi et al. 2013; Stafiej and Pyrzynska 2008). In fact, the number of available adsorption sites will increase by increasing the MWCNTs adsorbent dosage which will result in increased nickel uptake (Ajmal et al. 2000). The removal of nickel decreased as the MWCNTs concentration was increased beyond the optimum; this may be due to the overlapping of the adsorption sites and overcrowding of the adsorbent (Aregawi and Mengistie 2013). The decrease in the adsorption capacity, qe, is because the adsorption active sites remain unsaturated during the adsorption process (Singh and Rattan 2011; Filipović-Kovačević et al. 2000).
The effect of contact time and the initial Ni(II) ion concentration
Figure 3 shows the removal of Ni(II) at different contact times (5, 10, 25, and 40 min) and initial Ni(II) concentrations (25, 50, 100, and 125 mg/L) at optimum pH of 10 and optimum MWCNTs dosage of 10 mg/L. Maximum efficiency of Ni(II) removal was obtained at the time of 10 min for all concentrations. After this time, the removal efficiency declined. The study of the effect of the initial Ni(II) concentration of 25–125 mg/L revealed that the adsorption efficiency of the metal decreased with increasing initial concentration from 100 to 125 mg/L. The highest removal efficiency (82%) was observed for the concentration of 100 mg/L which was obtained as the optimum concentration at the time of 10 min. This is because, during the adsorption process, the surface of the adsorbent was easily saturated by the adsorbate molecules with higher dye concentration (Zhang and Wang 2015). But when the concentration was increased from 25 to 100 mg/L, the removal efficiency increased because the increase in the amount of Ni(II) ions resulted in the increase in collision between the ions and the active sites on the adsorbent (Zhang et al. 2017). As the contact time increases, the adsorption efficiency decreases. Initially, the adsorption process was rapid and became slow until the saturation phase was reached, which lasted for 10 min (Zhang et al. 2010, Ajmal et al. 2000). The efficiency was reduced after 10 min due to the occurrence of the desensitization phenomenon (Stafiej and Pyrzynska 2008). A similar trend was obtained by Akpomie et al. (2015) for the effect of contact time on the removal of Ni(II) ions using montmorillonite. The removal efficiency was studied at contact time of 20 to 300 min at concentration of 100 mg/L, pH 6.0, adsorbent dose of 0.1 g, temperature of 300 K, and particle size of 100 μm; but after a time of 200 min, the removal of Ni(II) ions declined.
The adsorption kinetics study
Adsorption kinetics are defined as the uptake of adsorption with time. It is also applied to determine the controlling mechanism of adsorption processes including chemical reaction and mass transfer (Kumar et al. 2010). The adsorption kinetics experiment was carried out using the optimum MWCNTs dosage of 10 mg/L and optimum pH of 10 at different concentrations (25, 50, 100, and 125 mg/L) and times (5, 10, 25, and 40 min) at room temperature. The kinetic data were fitted into the Ho’s pseudo-second-order and Lagergren’s pseudo-first-order kinetic models. They were applied in two forms of linear and nonlinear equations (Han et al. 2009; Ho 2006). The linear and nonlinear forms of the kinetic equations are shown in Table 2. Root-mean-square error (RMSE), as well as the R2 statistics, was used to evaluate the nonlinear models. Smaller values of RMSE indicate higher fit by the model. The RMSE equation is given by Eq. 3 (Han et al. 2009):
where qc is the value that is calculated from the model fit, qe is calculated from the test elements, and p is the number of test elements.
The Lagergren and Ho linear models fitting to the adsorption kinetic data are also shown in Figs. 5, 6, 7 and 8. The calculated parameters of Lagergren and Ho adsorption kinetics for the linear and nonlinear model are shown in Tables 3 and 4, respectively. The results indicated that the Ho(I) linear models fitted into the kinetic data than the Ho(II) and Ho(III) linear models with a correlation coefficient, R2 of 0.996 at the optimum concentration of 100 mg/L. Therefore, it seems that using the different forms of the linear Ho equations was not necessary. Also, Ho(I) provided better R2 value than Lagergren linear model which suggests that the rate determining step of the process is chemisorption (Ahmadi and Igwegbe 2018; Igwegbe et al. 2016; Al-Ghouti et al. 2005).
Figures 4 and 9 show the Lagergren and Ho nonlinear models for Ni(II) adsorption on MWCNTs. As seen in Table 4, the nonlinear Ho model had the least RMSE and the highest R2 which implies its best fit to the measured data compared to the nonlinear Lagergren model. This also indicates that the nonlinear Ho model provided a better explanation of the adsorption of Ni(II) ions on MWCNTs. Generally, the results indicated that the nonlinear method of regression analysis provided better results based on fitting the experimental data with Ho model which was also proved by other authors (Ho and McKay 2002; Rahdar et al. 2018b).
Conclusions
In this study, the adsorption of nickel (Ni(II)) onto multi-walled carbon nanotubes (MWCNTs) has been investigated. The effect of pH, MWCNTs dosage, initial concentration of Ni(II), and contact time on the removal efficiency of Ni(II) ions was studied. The experimental kinetic adsorption data of Ni(II) removal by MWCNTs were fitted into the linear and nonlinear kinetic models (Ho’s pseudo-second order and Lagergren’s pseudo-first order). The results of this study indicate that the nanomaterial can remove Ni(II) in a very short time. Optimum conditions for the most efficient operation of the MWCNTs on Ni(II) removal were achieved at pH of 10, MWCNTs dose of 10 mg/L, concentration of 100 mg/L, and the contact time of 10 min which gave a removal efficiency of 82%. The Ho nonlinear model provided a better explanation about adsorption kinetic of Ni(II) removal by MWCNTs. The rate determining step of the adsorption process was found to be chemisorption.
References
Abdel-Ghani NT, El-Chaghaby GA, Helal FS (2015) Individual and competitive adsorption of phenol and nickel onto multiwalled carbon nanotubes. J Adv Res 6:405–415. https://doi.org/10.1016/j.jare.2014.06.001
Agarwal Sh, Tyagi I, Kumar Gupta V, Dehghani MH, Jaafari J, Balarak D, Asif M (2016) Rapid removal of noxious nickel(II) using novel γ-alumina nanoparticles and multi-walled carbon nanotubes: kinetic and isotherm studies. J Mol Liq 224:618–623. https://doi.org/10.1016/j.molliq.2016.10.032
Ahmadi S, Igwegbe CA (2018) Adsorptive removal of phenol and aniline by modified bentonite: adsorption isotherm and kinetics study. Appl Wat Sci 8:170. https://doi.org/10.1007/s1320
Ahmadi Sh, Kord Mostafapour F (2017a) Adsorptive removal of aniline from aqueous solutions by Pistacia atlantica (Baneh) shells: isotherm and kinetic studies. J Sci Technol Environ Inform 5:327–335
Ahmadi S, Kord Mostafapour F (2017b) Survey of efficiency of dissolved air flotation in removal of penicillin G from aqueous solutions. Brit J Pharm Res 15:1–11. https://doi.org/10.9734/BJPR/2017/31180
Ahmadi Sh, Kord Mostafapour F (2017c) Tea wastes as a low cost adsorbent for the removal of COD from landfill leachate: Kinetic Study. J Sci Eng Res 4:103–164
Ahmadi S, Kord Mostafapour F (2017d) Treatment of textile wastewater using a combined Coagulation and DAF processes, Iran, 2016. Arch Hyg Sci 6:229–234. http://jhygiene.muq.ac.ir/article-1-272-en.html
Ahmadi S, Banach A, Kord Mostafapour F, Balarak D (2017) Study survey of cupric oxide nanoparticles in removal efficiency of ciprofloxacin antibiotic from aqueous solution: adsorption isotherm study. Desal Water Treat 89:297–303. https://doi.org/10.5004/dwt.2017.21362
Ahmadi S, Mohammadi L, Igwegbe CA et al (2018) Application of response surface methodology in the degradation of Reactive Blue 19 using H2O2/MgO nanoparticles advanced oxidation process. Int J Ind Chem 9:241. https://doi.org/10.1007/s40090-018-0153-4
Ajmal M, Rao RAK, Ahmad R, Ahmad J (2000) Adsorption studies on Citrus reticulata (fruit peel of orange): removal and recovery of Ni(II) from electroplating wastewater. J Hazard Mater 79:117–131. https://doi.org/10.1016/S0304-3894(00)00234-X
Akpomie KG, Dawodu FA, Adebowale KO (2015) Mechanism on the sorption of heavy metals from binary-solution by a low cost montmorillonite and its desorption potential. Alexandria Eng J 54:757–767. https://doi.org/10.1016/j.aej.2015.03.025
Al-Ghouti M, Khraisheh MAM, Ahmad MNM, Allen S (2005) Thermodynamic behavior and the effect of temperature on the removal of dyes from aqueous solution using modified diatomite: a kinetic study. J Colloid Interface Sci 287:6–13. https://doi.org/10.1016/j.jcis.2005.02.002
APHA, Wpcf (2005) Standard method for the examination of water and wastewater, 21st edn. Washingon, D.C
Aregawi BH, Mengistie AA (2013) Removal of Ni(II) from aqueous solution using leaf, bark and seed of Moringa stenopetala adsorbents. Bull Chem Soc Ethiop 27:35–47. https://doi.org/10.4314/bcse.v27i1.4
Banerjee S, Chattopadhyaya MC (2017) Adsorption characteristics for the removal of a toxic dye, tartrazine from aqueous solutions by a low cost agricultural by-product. Arab J Chem 10:S3381–S3393. https://doi.org/10.1016/j.arabjc.2013.06.005
Filipović-Kovačević Ž, Sipos L, Briski F (2000) Biosorption of chromium, copper, nickel and zinc ions onto fungal pellets of Aspergillus niger from aqueous solutions. Food Technol. Biotechnol 38:211–216
Han RJ, Zhang P, Han Y, Wang Z, Zhao M (2009) Study of equilibrium, kinetic and thermodynamic parameters about ethylene blue adsorption onto natural zeolite. Chem Eng J 145:496–504. https://doi.org/10.1016/j.cej.2008.05.003
Heidari Z, Masrournia M, Sannavii-Khoshnood R (2016) Fabrication a composite electrode based on MWCNT/Zeolite for potentiometric determination of Cr. Orient J Chem 32:627–635. https://doi.org/10.13005/ojc/320171
Ho Y (2006) Second-order kinetic model for the sorption of cadmium onto tree fern: a comparison of linear and non-linear methods. Water Res 40:119–125. https://doi.org/10.1016/j.watres.2005.10.040
Ho YS, McKay G (2002) Application of kinetic models to the sorption of copper (II) on to peat. Adsorpt Sci Technol 20:797–815. https://doi.org/10.1260/026361702321104282
Igwegbe CA, Onukwuli OD, Nwabanne JT (2016) Adsorptive removal of vat yellow 4 on activated Mucuna pruriens (velvet bean) seed shells carbon. Asian J Chem Sci 1:1–16. https://doi.org/10.9734/AJOCS/2016/30210
Igwegbe CA, Banach AM, Ahmadi S (2018) Adsorption of Reactive Blue 19 from aqueous environment on magnesium oxide nanoparticles: kinetic, isotherm and thermodynamic studies. Pharm Chem J 5:111–121
Kashitarash Esfahani Z, Samadi MT, Alavi M, Manuchehrpoor N, Bakhani M (2012) Efficiency of carbon nano tubes in municipal solid waste landfill leachate treatment (case study: Hamadan landfill leachate. J Water Wastewater 82:67–72
Kumar PS, Vincent C, Kirthika K, Kumar KS (2010) Kinetics and equilibrium studies of Pb2+ ion removal from aqueous solutions by use of nano-silver sol-coated activated carbon. Braz J Chem Eng 27:339–346. https://doi.org/10.1590/S0104-66322010000200012
Li YH, Zhao YM, Hu WB, Ahmad I, Zhu YQ, Peng XJ, Luan ZK (2007) Carbon nano tubes the promising adsorbent in wastewater treatment. J Phys 61:698–702
Rahdar S, Igwegbe CA, Rahdar A, Ahmadi S (2018a) Efficiency of sono-nano-catalytic process of magnesium oxide nanoparticle in the removal of penicillin G from aqueous solution. Desal Water Treat 106:330–335. https://doi.org/10.5004/dwt.2018.22102
Rahdar S, Shikh L, Ahmadi S (2018b) Removal of Reactive Blue 19 dye using a combined sonochemical and modified Pistachio shell adsorption processes from aqueous solutions. Iran J Health Sci 6:8–20. http://jhs.mazums.ac.ir/article-1-577-en.html
Salam MA, Makki MSI, Abdelaal MYA (2011) Preparation and characterization of multi-walled carbon nanotubes/chitosan nanocomposite and its application for the removal of heavy metals from aqueous solution. J Alloys Compd 509:2582–2587. https://doi.org/10.1016/j.jallcom.2010.11.094
Samadi MT, Kashitarash Esfahani Z, Ahangari F, Ahmadi Sh, Jafari J (2013) Nickel removal from aqueous environments using carbon nanotubes. J Water Wastewater 24:38–44
Sheng GD, Shao DD, Ren XM, Wang XQ, Li JX, Chen YX, Wang XK (2010) Kinetics and thermodynamics of adsorption of ionizable aromatic compounds from aqueous solutions by as—prepared and oxidized multiwalled carbon nanotubes. J Hazard Mater 178:505–516. https://doi.org/10.1016/j.jhazmat.2010.01.110
Singh H, Rattan VK (2011) Adsorption of nickel from aqueous solutions using low cost biowaste adsorbents. Water Qual Res J Can 46:239–249. https://doi.org/10.2166/wqrjc.2011.024
Stafiej A, Pyrzynska K (2008) Solid phase extraction of metal ions using carbon nanotubes. Micro Chem J 89:29–33. https://doi.org/10.1016/j.microc.2007.11.001
Zhang X, Wang X (2015) Adsorption and desorption of Nickel(II) ions from aqueous solution by a lignocellulose/montmorillonite nanocomposite. Water and modification of lignocellulose. PLoS ONE 10:e0117077. https://doi.org/10.1371/journal.pone.0117077
Zhang H, Tang Y, Cai D, Liu X, Wang X, Huang Q et al (2010) Hexavalent chromium removal from aqueous solution by algal bloom residue derived activated carbon: equilibrium and kinetic studies. J Hazard Mater 181:801–808. https://doi.org/10.1016/j.jhazmat.2010.05.084
Zhang X, Wang X, Chen Z (2017) A novel nanocomposite as an efficient adsorbent for the rapid adsorption of Ni(II) from aqueous solution. Materials 10:1124. https://doi.org/10.3390/ma10101124
Acknowledgements
The authors are most grateful to the laboratory staff of the Department of Environmental Health Engineering, Hamedan University of Medical Sciences, for financial support and their collaboration in this research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Authors declare there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ahmadi, S., Igwegbe, C.A., Rahdar, S. et al. The survey of application of the linear and nonlinear kinetic models for the adsorption of nickel(II) by modified multi-walled carbon nanotubes. Appl Water Sci 9, 98 (2019). https://doi.org/10.1007/s13201-019-0978-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-019-0978-9