Abstract
Awash River, one of the major rivers in Ethiopia which originate from the central highland area, crosses different extended agricultural farms as well as various industries which receives large effluents from different sources. Nineteen physicochemical parameters, as well as heavy metals (Fe, Zn, Cu, Pb, Cr, Cd and Ni), were quantified using atomic absorption spectroscopy in eight different sampling stations of Awash River. There was a significant spatial variation (p < 0.05) in average NO3-N, NH4-N, TN, BOD and COD values in Awash River. There is a strong and positive correlation between (pH and EC, r = 0.805), (WT and BOD, r = 0.774), (NO3-N and NO2-N, r = 0.901), (NO3-N and TN, r = 0.906), (NO3-N and TP, 0.830), (NH4-N and TN, r = 0.876), (NH4-N and COD, r = 0.848), (TN and TP, r = 0.819), (TN and COD, r = 0.941) during dry season and also between (WT and BOD, r = 0.704), (turbidity and NO3-N, r = 0.749), (turbidity and NO2-N, r = 0.722), (NO3-N and NO2-N, r = 0.921), (TP and COD, r = 0.789) during wet season. The results showed that the mean concentrations of metals ranked (high to low) Fe > Cr > Cu > Zn > Pb > Cd > Ni during dry season, whereas the concentration of heavy metals during wet season was in the following order of decreasing magnitude Fe > Cu > Zn > Pb > Cr > Cd > Ni. Buffer zones should be protected in order to control soil and agricultural nutrients from entering to Awash River. Moreover, industries at the upper stream area should be properly and adequately treat the wastewater before discharging to the Modjo as well as Awash River.
Similar content being viewed by others
Introduction
Currently, surface water pollution has received much attention globally. Both natural process and anthropogenic activities, like hydrological features, climate change, precipitation, agricultural activities, and wastewater discharge from industries, are the main reason for worsening of surface water quality (Ravichandran 2003; Gantidis et al. 2007; Arain et al. 2008).
Surface water mainly rivers has different purposes in various sectors like agriculture, industry, transportation, and domestic water supply. Nonetheless, rivers have also been used for cleaning and dumping purposes. This practices more prominent in developing countries, mostly in Africa. Wastewater from industries, domestic sewage, and agricultural farms is discharged into rivers which leads to deteriorate surface water quality (Ravindra et al. 2003). Rivers are among the main vulnerable aquatic environment to pollution because of far flow to take municipal, industrial wastes and agrochemicals through runoff (Singh et al. 2005).
Surface water quality in various areas is largely affected by both natural process (precipitation and weathering process) and anthropogenic activities like domestic sewage, industrial pollution, and agricultural activities (Varol et al. 2011). Domestic sewage and industrial wastewater discharge is a point source of pollution, whereas agricultural pollution is a nonpoint source of pollution through surface runoff and varies with season depending on the climatic condition of the specific region (Singh et al. 2004). The concentration of contaminant in river water changes with season as a result of variation in precipitation (Vega et al. 1998).
Nutrients in surface water have been mainly related with land use activities (Howarth 1988). Anthropogenic activities of point and nonpoint source of pollution are the major causes for nutrient enrichment of surface water.
Municipal sewage and industrial wastewater are the point source of nutrient pollution in aquatic environment, whereas inorganic fertilizers in agricultural fields and animal manure are nonpoint source of pollution which are responsible for nutrient enrichment in aquatic environment (Capone and Kiene 1988).
Surface water pollution by heavy metals is the main concern due to the toxicity and persistent nature as well as bioaccumulation effect in the environment (Sin et al. 2001; Cook et al. 1990). Heavy metals drain into a river from various sources, either natural or anthropogenic (Adaikpoh et al. 2005; Akoto et al. 2008). Usually in nonpolluted environments, the level of heavy metals in rivers is insignificant and mostly originates from rock and soil weathering (Reza and Singh 2010). The main anthropogenic sources of heavy metal in rivers are raw wastewater from industries, mining activities, sewage, and agrochemicals from agricultural fields (Macklin et al. 2006; Martin 2000; Nouri et al. 2008; Reza and Singh 2010).
Awash River, one of the major rivers in Ethiopia which originate from the central highland area crosses different extended agricultural farms as well as various industries which receives large effluents from different sources (Tesfamariam 1989). In the country, all of the prevailing industries and main town with in the upper watershed have no proper treatment plants resulting in polluting the river (MWEE 2010).
Furthermore, the Modjo River, which is susceptible for pollution due to discharging of wastewater from tannery industries, is the main tributary of Awash River. In addition, discharging of wastewater from different industries to the Awash River as well as industrial development is of the major problem of the country (Girma 2001).
Previously, there has not been any work on spatial and seasonal variation in physicochemical parameters and heavy metal in Awash River. The aim of this study was therefore to evaluate the level of different physicochemical parameters and heavy metals in terms of space and season in Awash River.
Materials and methods
Study area
The Awash River is the most important river in Ethiopia and serves as home to 10.5 million inhabitants. The river rises on the high plateau near Ginchi town west of Addis Ababa in Ethiopia and flows along the rift valley into the Afar triangle and terminates in salty Lake Abbe on the border with Djibouti. The total length of the main course is some 1200 km.
Water sampling
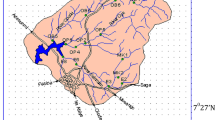
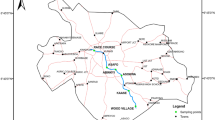
Sampling strategy was designed to cover a wide range of physiochemical parameters and heavy metals at sampling sites in Awash River. Water sampling was carried out on seasonal basis, namely during dry season (March–May, 2015) and rainy season (June–August, 2015). A total of 48 water samples were collected from eight sampling stations (24 samples during rainy season and 24 during dry season). Sampling, preservation, and transportation of the water samples to the laboratory were as per standard method (APHA 1998) (Fig. 1).
Analysis of water samples
The samples were analyzed for 19 parameters, namely water temperature (WT), pH, electrical conductivity (EC), turbidity, nitrate nitrogen (NO3-N), nitrite nitrogen (NO2-N), ammonia nitrogen (NH4-N), total nitrogen (TN), total phosphorus (TP), dissolved oxygen (DO), biological oxygen demand (BOD), chemical oxygen demand (COD), iron (Fe), zinc (Zn), copper (Cu), lead (Pb), chromium (Cr), Cadmium (Cd), and nickel (Ni). pH was measured on the sampling sites by pH meter model 370. WT and EC were also determined in the field using conductivity meter model CON 2700. All other parameters were determined in the laboratory following standard protocols (APHA 1995). TN (persulfate digestion method), NO3-N (cadmium reduction method), NO2-N (diazotization method), NH3-N (Nessler method), TP (persulfate digestion followed by ascorbic acid method), COD (dichromate reactor digestion method) were analyzed by HACH DR/2400, whereas Fe, Zn, Cu, Pb, Cr, Cd, and Ni were analyzed using graphite atomic absorption spectrometer. Each analysis was performed in triplicate, and the mean value was taken. The analytical data quality was guaranteed through the implementation of laboratory quality assurance and quality control methods, including the use of standard operating procedures, calibration with standards, analysis of reagent blanks, recovery of known additions, and analysis of replicates.
Statistical analysis
Statistical analysis was performed by SPSS version 16.0 to calculate average mean, standard deviation, and Pearson’s correlation (r) value to show the degree of physicochemical and metal association in river water. The ANOVA test (level of significance α = 0.05) was employed to understand the spatial and seasonal variation in the physico-chemical and heavy metal concentrations.
Result and discussion
Seasonal and spatial variation in physicochemical parameters
The concentration of physicochemical parameters in dry and wet season of Awash River is shown in Tables 1 and 2. During the study period, water temperature in Awash River showed some seasonal variation and ranged from 19.1 to 23.6 °C. As expected, water temperature was the highest during dry seasons and the lowest during wet seasons. The highest average water temperature values were recorded at site 7 during both dry season (23.01 °C) and wet season (21.9 °C). The reason might be there has been drinking water treatment plant at sampling station 7 so that the wastewater which drains from the treatment plant makes the river water temperature rise. There is no significant variation in water temperature among the sampling sites (p > 0.05), while there was a significant difference in seasonal mean concentration of water temperature (p < 0.05).
The mean water temperature value (22.2 °C) in the present study was higher than the average value (16.7 °C) in Tinishu Akaki River, Ethiopia, reported by Samuel et al. (2007), but it was substantially lower than the mean water temperature value (25.65 °C) in Upper Awash River, Ethiopia (Fasil et al. 2013).
Mean pH values at all sampling stations were slightly acidic to alkaline. The pH ranged from 6.08 to 8.47. Site 6 showed higher pH value (8.45) during the dry season. The lowest pH value (6.08) was found at site 7 in dry season. The lowest pH might be the sludge from drinking treatment plant mainly aluminum sulfate which lowers the pH of the river water. The deposition of sediment at Koka reservoir (site 6) is responsible for pH elevation. There is a significant variation in mean pH value among the sampling sites in Awash River (p < 0.05), while there was no seasonal significant difference in mean pH value in Awash River.
The average pH value (7.23) in the present study is lower than the mean value (8.44) reported from Guder River, Ethiopia (Bizualem 2017), and in Upper Awash River, Ethiopia (8.33) (Fasil et al. 2013), but higher than the mean pH (6.54) value of Buriganga River, Bangladesh (Ahmmad et al. 2016), Iguedo River, Edo State, Nigeria (5.65) (Udebuana et al. 2014).
The turbidity values in Awash River varied from 29.27 to 159.51 NTU (Tables 1 and 2). The highest mean turbidity values (139.61 NTU) were found at site 2 during wet season because of surface runoff from nearest agricultural land, and the lowest average value (36.4 NTU) of turbidity was recorded at sampling site 6 during dry season. Higher values were recorded during the raining season as compared to the dry season. This could be attributed to run off water from the agricultural farm which carries suspended materials into the river. The soil around Koka area is bare and hence highly susceptible to erosion during rainy seasons. Sampling sites 2, 3, and 4 had higher turbidity levels than the rest of the sampling sites.
There is a significant spatial and seasonal variation (p < 0.05) in average turbidity value among sampling sites (Table 3). The mean turbidity value in Awash River during rainy season (121.06 NTU) was substantially higher than the value of turbidity (57 NTU) in Walgamo River, Ethiopia (Dessalew et al. 2017), in Gudbahi River, Eastern Tigray, Ethiopia (9.6 NTU) (Mehari 2013).
The NO3-N concentration varied from 0.28 to 28.8 mg l−1. The highest mean concentration (27.87 mg l−1) of NO3-N was found at site 3 during dry season because of intensive agricultural activities near to this site and animal manure waste near the river. The lowest average concentration (0.48 mg l−1) of NO3-N was found at sampling site 1 during wet season. A significant variation in nitrate in the spatial trend was observed (p < 0.05). Nitrate is the most oxidized form of nitrogen found in aquatic environment, and during rainy season, considerable amount of nitrate washed from the agricultural farm and reached to water body through runoff.
The mean concentration of NO3-N (9.34 mg l−1) in Awash River was higher than the average value (3.74 mg l−1) from Jajrood River, Iran (Razmkhah et al. 2010), from Vishwamitri River, India (0.06 mg l−1) (Magadum et al. 2017), from Sinos River, Brazil (0.3 mg l−1) (Steffens et al. 2015), but substantially lower than the average NO3-N concentration (26.93 mg l−1) from Chambal River, Rajasthan, India (Gupta et al. 2011), from Mahanadi River, India (36.2 mg l−1) (Rout et al. 2016), from Ogun River, Nigeria (35.18 mg l−1) (Onozeyi 2013).
The NO2-N concentration varied from 0.06 to 0.92 mg l−1. The highest mean value (0.90 mg l−1) of NO2-N was reported at sampling site 3 during dry season, while the lowest mean concentration (0.07 mg l−1) was observed at sampling site 8 during wet season.
The mean value (0.42 mg l−1) of NO2-N concentration in the present study was higher than the average value (0.06 mg l−1) in Tigris River, Turkey (Varol et al. 2011), and also Elala River, Tigray, Ethiopia (0.11 mg l−1) (Ftsum et al. 2015), while it is considerably lower than the average value (1.07 mg l−1) in Awash River, Ethiopia (Amare et al. 2017).
The measured NH4-N values vary between 0.11 and 1.47 mg l−1 in dry season and between 0.03 and 0.35 mg l−1 in wet season. Site 4 showed higher average values (1.41 mg l−1) during dry season while the lowest NH4+ mean value (0.05 mg l−1) was found at site 1 in wet season. There is a significant spatial and seasonal variation (p < 0.05) in mean NH4-N values in Awash River (Table 3). NH4-N is a water-soluble gas that exists at low levels (0.1 mg l−1) in natural waters. NH4+ comes from the nitrogen-containing organic material and gas exchange between the water and the atmosphere (Chapman and Kimstach 1996). It also derives from the biodegradation of waste and from domestic, agricultural, and industrial wastes.
The mean value (0.78 mg l−1) of NH4-N in Awash River was higher than the average value (0.07 mg l−1) from Upper Awash River, Ethiopia (Fasil et al. 2013), Tigris River, Iraq (0.11 mg l−1) (Kadhem 2013).
The TN ranged from 0.82 to 84.53 mg l−1 (Tables 1 and 2). The highest mean values (83.43 mg l−1) of TN have been noted at sampling site 3 in dry season, and the lowest average concentration (1.22 mg l−1) was found at site 1 during wet season. There is a significant variation in mean TN values among sampling stations (p < 0.05); however, there was no seasonal significant difference in average TN concentration in Awash River.
The mean concentration (33.71 mg l−1) of TN in the present study was very similar to the average TN (35.21 mg l−1) in Walleme River, Ethiopia (Minuta and Jini 2017), but significantly higher than the mean TN value (2.06 mg l−1) in Tigris River, Turkey (varol et al. 2011), from Xin’anjing River, China (1.55 mg l−1) (Li et al. 2014).
The concentration of TP varied from 0.02 to 0.31 mg l−1 in dry season and between 0.03 and 0.28 mg l−1 in wet season. Site 3 showed higher mean values (0.27 mg l−1) during dry season while the lowest average TP value (0.04 mg l−1) was found at site 7 in dry season. There was no a significant spatial and seasonal variation (p > 0.05) in average TP values in Awash River (Table 3).
The DO values varied from 3.02 to 13.51 mg l−1. The DO was higher in wet season than in dry season at almost all sites. The low DO values in dry months were possibly due to considerable activities of microorganisms, which consumed appreciable amount of oxygen as a result of metabolizing activities and decay of organic matter. The highest mean values (10.82 51 mg l−1) of DO were observed at site 1 during wet season. The lowest concentration (3.62 mg l−1) of DO was found at site 4 during dry season, which receives agricultural runoff and animal manure wastes near the river. Dissolved oxygen is probably the most important parameter in natural surface water systems for determining the health of aquatic ecosystems (Yang et al. 2007).
The average value (6.48 mg l−1) of DO in Awash River was very similar to the mean DO value (6.62 mg l−1) from Blue Nile River, Ethiopia (Abrehet et al. 2015), but considerably higher than the mean DO value (1 mg l−1) from Modjo River, Ethiopia (Abrha et al. 2015), from Mahanadi River, India (4.58 mg l−1) (Rout et al. 2016), from Ngong River, Kenya (4.35 mg l−1) (Mobegi et al. 2016).
The concentration of BOD varied from 13.69 to 83.37 mg l−1 in dry season and between 9.14 and 39.47 mg l−1 in wet season. Site 4 showed higher average values (80.32 mg l−1) of BOD during dry season while the lowest average BOD value (11.13 mg l−1) was found at site 1 in wet season (Tables 1 and 2). There was a significant spatial variation (p < 0.05) in average BOD values in Awash River, whereas there was no significant seasonal variation (p > 0.05) in mean BOD values among the sampling sites (Table 3).
Based on the result of the present study, average BOD value (37.49 mg l−1) was significantly higher than the mean value of BOD (24.23 mg l−1) from Nyabugogo catchment, Rwanda (Nhapi et al. 2011), Gudbahri River, Eastern Tigray, Ethiopia (3.88 mg l−1) (Mehari 2013), Rapti River, India (34.33 mg l−1) (Chaurasia and Tiwari 2011), but lower than the mean value (38.10 mg l−1) of BOD from Nile River, Egypt (Elewa 2010).
COD in Awash River varied from 16.13 to 150.38 mg l−1. The highest average COD values (147.98 mg l−1) were found at site 3 during dry season because of different agrochemicals’ discharge to the river through runoff. The lowest mean value (19.08 mg l−1) of COD was recorded at sampling site 1 during wet season. The average COD values were indicated a significant spatial variation (p < 0.05) among the sampling sites, but there was no seasonal variation in mean COD values in Awash River (Table 3). High values of COD indicate water pollution, which is associated with wastewater discharged from industry or agricultural practices (Bellos and Sawidis 2005).
The mean value (76.82 mg l−1) of COD in Awash River was substantially lower than the average concentration (651 mg l−1) of COD from Modjo River, Ethiopia (Abrha et al. 2015), from Buniganga River, Bangladesh (Ahmmad et al. 2016).
The covariance matrix of the 12 analyzed variables was calculated from normalized data; consequently, it coincided with the correlation matrix (Tables 4 and 5). Because the eight sampling stations were combined to determine the correlation matrix, the correlation coefficients should be interpreted; however, they are affected simultaneously by spatial and seasonal variation.
There is a strong and positive correlation between (pH and EC, r = 0.805), (WT and BOD, r = 0.774), (NO3-N and NO2-N, r = 0.901), (NO3-N and TN, r = 0.906), (NO3-N and TP, 0.830), (NH4-N and TN, r = 0.876), (NH4-N and COD, r = 0.848), (TN and TP, r = 0.819), (TN and COD, r = 0.941). A significant negative correlation exists between (WT and turbidity, r = −0.812), (WT and DO r = −0.927), (TN and BOD, r = −0.854) during dry season (Table 4).
Strong and positive correlations exist between (WT and BOD, r = 0.704), (turbidity and NO3-N, r = 0.749), (turbidity and NO2-N, r = 0.722), (NO3-N and NO2-N, r = 0.921), (NO3-N and BOD, 0.832), (TP and COD, r = 0.789). A significant negative correlation exists between (WT and NH4-N, r = −0.769) during wet season. The positive correlation probably indicated that these pollutants came from the same sources that are from agricultural runoff and animal manure.
Seasonal and spatial variation in heavy metals
Concentrations of heavy metals in water from each sampling site are given in Tables 6 and 7. The highest mean concentration of Fe during dry season was at site 5 at, 2.73 mg l−1, with values ranging from 1.85 to 3.87 mg l−1 while the lowest mean concentration of it was measured at site 1 at 1.11 mg l−1, with values ranging from 0.49 to 1.64 mg l−1. There is a fluctuation in the spatial variations during wet season with minimum average concentration of 1.82 mg l−1 at site 1 with the highest mean concentration of 4.12 mg l−1 occurring at station 5. There were no significant differences (p < 0.05) in Fe concentrations among the sampling sites. Nevertheless, the seasonal trends in the distribution of Fe showed significant changes (p < 0.05) (Table 8).
The average concentrations of Fe (1.11–4.12 mg l−1) in the present study were significantly higher than the level of Fe in Sosiani River reported in Kenya (0.011–2.897 ppm) (Amadi 2013), but substantially lower than the mean Fe concentrations (12.6–15.51 mg l−1) in Mara River, Tanzania (Kihampa and Wenaty 2013).
The highest mean concentration of Zinc during dry season was measured at site 5 at, 1.56 mg l−1, with values ranging from 0.47 to 2.95 mg l−1 while the lowest mean concentration of Zinc was measured at site 1 at 0.74 mg l−1, with values ranging from 0.35 to 1.46 mg l−1. There is a variation in Zinc concentration during wet season with the lowest mean value of 0.46 mg l−1 at site 8 with maximum average concentration of 0.91 mg l−1 at sampling station 5 (Table 7). There was a significant seasonal variation (p < 0.05) in Zn concentrations. On the other hand, there was no significant difference in zinc concentration among the sampling station (Table 8).
The present study showed that the average Zn level (0.46–1.56 mg l−1) measured in Awash River was higher than the River Nile from Egypt (0.12–0.69 ppm) (Osman and Kloas 2010), but lower than the Zn concentrations (0.96–2.14 mg l−1) from Kampani River, Plateau State, Nigeria (Lawal et al. 2014). Zinc is an indispensible trace element not only for human, but also for all organisms. It is a component of proteins as well as greater number of enzymes (Plum et al. 2010). High concentration of zinc leads phytotoxicity, reproduction problem, and brain disorder (USEPA 1999).
The average concentration of Cu during dry season ranged from 0.82 to 1.69 mg l−1: The highest concentration of Cu during dry season was recorded at site 4 while the lowest average concentration of Cu was measured at site 8. The mean concentration of Cu during wet season ranged from 0.44 to 1.01 mg l−1: The highest concentration of Cu during dry season was recorded at site 4 while the lowest average concentration of Cu was measured at site 8. The seasonal trend of Cu showed significant variations (p < 0.05). However, the overall spatial variations showed no significant changes (Table 8).
The present study revealed that the mean Cu level (0.44–1.69 mg l−1) in Awash River was higher than the level reported in Dzindi River (0.03–0.05 mg l−1), from Limpopo Province, South Africa (Edokpayi et al. 2016), but lower than the mean Cu concentrations (2.99–4.90 mg l−1) in dam water from Nairobi, Kenya (Ndeda and Manohar 2014).
The average concentrations of Pb were slightly variable between sampling points. The value of Pb ranged 0.41–1.36 mg l−1 during dry season. The highest concentration of Pb during dry season was detected at site 5 while the lowest mean concentration of Pb was recorded at site 8. The mean concentration of Pb during wet season ranged from 0.31 to 0.83 mg l−1: The highest concentration of Pb during wet season was recorded at site 5, whereas the lowest average concentration of Pb was measured at site 8. The seasonal and the spatial mean concentration levels of Pb were not significantly different (p > 0.05) (Table 8).
The mean concentration of Pb (0.31–1.36 mg l−1) in river water of the present study was found higher than the values (0.05–0.67 ppm) reported by Mutembei et al. (2014) in Naka River, Kenya. Lead is a nonessential and toxic metal which is usually associated with various diseases like memory lapses, anemia, anorexia, constipation. High concentrations of lead are known to cause death or permanent damage to the central nervous system, the brain, and kidneys when absorbed in humans (Jennings et al. 1996).
The mean concentration of Cr ranged 0.36–1.16 mg l−1 during dry season. The highest concentration of Cr during dry season was measured at site 5, and the lowest average concentration of Cr was recorded at sampling site 1. The mean concentration of Cr during wet season ranged from 0.30 to 0.98 mg l−1. The highest concentration of Cr during wet season was measured at site 6, and the lowest average concentration of Cr was recorded at sampling site 1.
The mean concentration of Cr (0.30–1.16 mg l−1) in river water recorded during the present study was substantially lower than the average Cr concentration (1.49–3.16 mg l−1) in Niger River, Nigeria (Olatunji and Osibanjo 2012).
The highest mean concentration of cadmium during dry season was measured at site 6 at, 0.24 mg l−1, with values ranging from 0.18 to 0.29 mg l−1, while the lowest mean concentration of cadmium was measured at site 8 at 0.05 mg l−1, with values ranging from 0.04 to 0.07 mg l−1. There is a variation in cadmium concentration during wet season with the lowest value of 0.03 mg l−1 at site 8 with maximum concentration of 0.11 mg l−1 at sampling station 5 (Table 7).
The mean concentration of Cd (0.03–0.24 mg l−1) in the present study was substantially higher than the level reported in Sosiani River (0.003–0.05 ppm) from Kenya (Amadi 2013) and Thohoyandou, South Africa (1.6–3.3 µg l−1) (Okonkwo and Mothiba 2005), but lower than the average Cd concentrations (3.76–5.12 mg l−1) in dam water from Nairobi, Kenya (Ndeda and Manohar 2014).
The highest mean concentration of Nickel during dry season was measured at site 6 at, 0.2 mg l−1, with values ranging from 0.16 to 0.25 mg l−1, whereas the lowest average concentration of Nickel was measured at site 8 at 0.03 mg l−1, with values ranging from 0.02 to 0.05 mg l−1. There is a difference in average nickel concentration during wet season with the lowest value 0.02 mg l−1 at site 8 with maximum mean value of 0.09 mg l−1 at sampling station 6.
The average concentrations of Ni (0.02–0.2 mg l−1) in Awash River were significantly lower than the level of Ni (1.2–2.11 mg l−1) in dam water from Nairobi, Kenya (Ndeda and Manohar 2014).
The results showed that the mean concentrations of metals ranked (high to low): Fe > Cr > Cu > Zn > Pb > Cd > Ni during dry season, whereas the concentration of heavy metals during wet season was in the following order of decreasing magnitude Fe > Cu > Zn > Pb > Cr > Cd > Ni (Fig. 2). The concentration of heavy metals during dry season was higher than the wet season except for Fe in which the highest concentration was found during wet season. The highest concentration of Fe during wet season attributed to high runoff during rainy season eroded the soil particles containing iron. Whereas the highest concentration of most of the metals during dry season is due to more gentle flow of the river during the dry season and water volume had reduced during the dry season making the dissolved metals to be at higher concentration levels in the liquid phase.
Matrices of correlation coefficient between the metal levels in the water are presented in Tables 9 and 10 for the dry and wet seasons, respectively. Strong and positive correlations exist between (Fe/Zn, r = 0.804), (Fe/Pb, r = 0.803), (Fe/Cr, r = 0.824), (Fe/Cd, r = 0.775), (Fe/Ni, r = 0.825), (Zn/Cr, r = 0.705), (Zn/Cd, r = 0.90), (Zn/Ni, r = 0.741), (Pb/Cr, r = 0.712), (Cr/Cd, r = 0.849), (Cr/Ni, r = 0.812), (Cd/Ni, r = 0.882) during dry season (Table 9). Moreover, in wet season there is also strong correlation among most of the heavy metals.
The results showed significant direct correlation between most of the metals at p < 0.05. This may be due to the existence of some of these metals in similar oxidation state reacting in the same manner to the aqueous environment or that the metals with high correlation coefficient exist together in a mineral and are leached into the aquatic system (Asaolu 1998; Aiyesanmi 2006). Furthermore, the strong association between most of the metals indicated that their common sources might be surface runoff of agrochemicals from agricultural fields and also wastewater discharge from the upstream industries.
Conclusion
There is a significant spatial and seasonal variation in most of the physicochemical parameters in Awash River. The concentration of heavy metals during dry season is higher than the wet season except for Fe in which the highest concentration was found during wet season. Matrices of correlation coefficient indicated significant direct correlation between most of the metals at (p < 0.05) for the dry and wet season. Intensive application of inorganic fertilizers like urea, DAP, and pesticides at Koka and Wonji farm site needs to be controlled by concerned bodies since these agrochemicals are the source of heavy metal pollution and eutrophication in Awash River. Buffer zones should be protected in order to control soil and agricultural nutrients from entering to Awash River. Moreover, industries at the upper stream area should be properly and adequately treat the wastewater before discharging to the Modjo as well as Awash River and environmental protection agency need to regularly monitor and test the wastewater based on the standard guidelines.
References
Abrehet KM, Shewit G, Belayneh A (2015) Effects of Bahir Dar textile factory effluents on the water quality of the head waters of Blue Nile River, Ethiopia. Int J Anal Chem. https://doi.org/10.1155/2015/905247
Abrha M, Tenalem A, Shifare B (2015) Impact of slaughterhouses effluent on water quality of Modjo and Akaki River in Central Ethiopia. Int J Sci Res 4(3):899–907
Adaikpoh EO, Nwajei GE, Ogala JE (2005) Heavy metals concentrations in coal and sediments from River Ekulu in Enugu, Coal City of Nigeria. J Appl Sci Environ Manag 9:5–8
Ahmmad SS, Tasfina S, Rabbani KA, Khaleque A (2016) An investigation into the water quality of Buriganga: a river running through Dhaka. Int J Sci Technol Res 5(3):36–41
Aiyesanmi AF (2006) Baseline concentration of heavy metals in water samples from rivers within Okitipupa southeast belt of the Nigerian bitumen field. J Chem Soc Niger 31(1&2):30–37
Akoto O, Bruce TN, Darko G (2008) Heavy metals pollution profiles in streams serving the Owabi reservoir. Afr J Environ Sci Technol 2:354–359
Amadi EK (2013) Nutrient loads and heavy metals assessment along Sosiani River, Kenya. Chem Mater Res 3(12):14–20
Amare SK, Zebene K, Agizew NE (2017) Evaluating water quality of Awash River using water quality index. Int J Water Resour Environ Eng 9(11):243–253
American Public Health Association (APHA) (1995) Standard methods for examination of water and wastewater, 20th edn. American Public Health Association, Washington
American Public Health Association (APHA) (1998) Standard methods for examination of water and wastewater, 20th edn. American Public Health Association, Washington
Arain MB, Kazi TG, Jamali MK, Jalbani N, Afridi HI, Shah A (2008) Total dissolved and bioavailable elements in water and sediment samples and their accumulation in Oreochromis mossambicus of polluted Manchar Lake. Chemosphere 70:1845–1856
Asaolu SS (1998) Chemical pollution studies of Coastal Waters of Ondo State, Nigeria. PhD thesis, Federal University of Technology, Akure
Bellos D, Sawidis T (2005) Chemical pollution monitoring of the river Pinios (Thessalia-Greece). J Environ Manag 76:282–292
Bizualem W (2017) Characterization of physicochemical water quality parameters of River Gudar (Oromia Region, West Shewa Zone, Ethiopia) for drinking purpose. J Appl Chem 10(5):47–52
Capone DG, Kiene RP (1988) Comparison of microbial dynamics in marine and freshwater sediments: contrasts in anaerobic carbon catabolism. Limnol Oceanogr 33:725–749
Chapman D, Kimstach V (1996) Selection of water quality variables. In: Chapman D (ed) Water quality assessments: a guide to the use of biota, sediments and water in environment monitoring (2188–2187), 2nd edn. E FN Spon, London, pp 59–126
Chaurasia NK, Tiwari RK (2011) Effect of industrial effluents and wastes on physico-chemical parameters of river Rapti. Adv Appl Sci Res 2(5):207–211
Cook JA, Andrew SM, Johnson MS (1990) Lead, zinc, cadmium and fluoride in small mammals from contaminated grass-land established on fluorspar tailings. Water Air Soil Pollut 51:43–54
Dessalew B, Belina T, Getachew D (2017) Assessment on the current water quality status of Walgamo River, Addis Ababa, Ethiopia. Int J Innov Res Sci Eng Technol 6(8):1–12
Edokpayi JN, Odiyo JO, Popoola OE, Msagati TAM (2016) Assessment of trace metals contamination of surface water and sediment: a case study of Mvudi River, South Africa. Sustainability 8(35):1–13
Elewa HH (2010) Potentialities of water resources pollution of the Nile River Delta, Egypt. Open Hydrol J 4:1–13
Fasil D, Aschalew L, Yared T, Kibru T (2013) The water quality degradation of Upper Awash River, Ethiopia. Ethiop J Environ Stud Manag 6(1):58–66
Ftsum G, Abraha G, Amanual H, Samuael E (2015) Investigations of physico-chemical parameters and its pollution implications of Elala River, Mekelle, Tigray, Ethiopia. Momona Ethiop J Sci 7(2):240–257
Gantidis N, Pervolarakis M, Fytianos K (2007) Assessment of the quality characteristics of two lakes (Koronia and Volvi) of N. Greece. Environ Monit Assess 125:175–181
Girma T (2001) Land degradation: a challenge to Ethiopia. Environ Manag 27:815–824
Gupta N, Nafees SM, Jain MK, Kalpana S (2011) Physico-chemical assessment of water quality of river Chambal in Kota City area of Rajasthan State (India). Rasayan J Chem 4(2):686–692
Howarth R (1988) Nutrient limitation of net plenary production in manne ecosystems. Annu Reverse Ecol Syst 19:89–110
Jennings GD, Sneed RE, Clair MB (1996) Metals in drinking water. North Carolina Coop Ext Service Publ 3:542–556
Kadhem AJ (2013) Assessment of water quality in Tigris River-Iraq by using GIS mapping. Nat Resour 4:441–448
Kihampa C, Wenaty A (2013) Impact of mining and farming activities on water and sediment quality of the Mara river basin, Tanzania. Res J Chem Sci 3(7):15–24
Lawal RA, Lohdip YN, Egila JN (2014) Water quality assessment of Kampani River, Plateau State, Nigeria. Asian Rev Environ Earth Sci 1(2):30–34
Li X, Li P, Wang D, Wang Y (2014) Assessment of temporal and spatial variations in water quality using multivariate statistical methods: a case study of the Xin’anjiang River, China. Front Environ Sci Eng 8(6):895–904
Macklin MG, Brewer PA, Hudson-Edwards KA, Bird G, Coulthard TJ, Dennis IA, Lechler PJ, Miller JR, Turner JN (2006) A geomorphological approach to the management of rivers contaminated by metal mining. Geomorphology 79:423–447
Magadum A, Patel T, Gavali D (2017) Assessment of physicochemical parameters and water quality index of Vishwamitri River, Gujarat, India. Int J Environ Agric Biotechnol 2(4):1505–1510
Martin CW (2000) Heavy metal trends in floodplain sediments and valley fill, River Lahn, Germany. CATENA 39:53–68
Mehari M (2013) Physico-chemical analysis of Gudbahri River Water of Wukro, Eastern Tigray, Ethiopia. Int J Sci Res Publ 3(11):1–4
Minuta T, Jini D (2017) Impact of effluents from wet coffee processing plants on the Walleme River of Southern Ethiopia. Res J Environ Toxicol 11(3):90–96
Mobegi EK, Nyambaka HN, Nawiri MP (2016) Physico-chemical characteristics and levels of polycyclic aromatic hydrocarbons in untreated water from Ngong River, Kenya. J Pollut Eff Control 4(2):1–4
Mutembei JK, Salim AM, Onditi OA, Waudo W, Yusuf AO (2014) Determination of heavy metals and nutrients in Rivers Naka and Irigu, Chuka, (Kenya) using atomic absorption spectrometry and UV/visible spectrophotometry. IOSR J Appl Chem 7(11):82–88
Ministry of Water and Energy of Ethiopia (MWEE) (2010) Water quality management. Addis Ababa, Ethiopia
Ndeda LA, Manohar S (2014) Determination of heavy metals in Nairobi Dam Water, (Kenya). J Environ Sci Toxicol Food Technol 8(5):68–73
Nhapi I, Wali UG, Uwonkunda BK, Nsengimana H, Banadda N, Kimwaga R (2011) Assessment of water pollution levels in the Nyabugogo Catchment, Rwanda. Open Environ Eng J 4:40–53
Nouri J, Mahvi AH, Jahed GR, Babaei AA (2008) Regional distribution pattern of ground water heavy metals resulting from agricultural activities. Environ Geol 55:1337–1343
Okonkwo JO, Mothiba M (2005) Physico-chemical characteristics and pollution levels of heavy metals in the rivers in Thohoyandou. S Afr J Hydrol 308:122–127
Olatunji OS, Osibanjo O (2012) Determination of selected heavy metals in inland fresh water of lower River Niger drainage in North Central Nigeria. Afr J Environ Sci Technol 6(10):4013–4408
Onozeyi DB (2013) Assessment of some physico-chemical parameters of River Ogun (Abeokuta, Ogun State, Southwestern Nigeria) in comparison with national and international standards. Int J Aquac 3(15):79–84
Osman AGM, Kloas W (2010) Water quality and heavy metal monitoring in water, sediments, and tissues of the African Catfish Clarias gariepinus (Burchell, 1822) from the River Nile, Egypt. J Environ Prot 1:389–400
Plum LM, Rink L, Haase H (2010) The essential toxin: impact of zinc on human health. Int J Environ Res Public Health 7:1342–1365
Ravichandran S (2003) Hydrological influences on the water quality trends in Tamiraparani Basin, south India. Environ Monit Assess 87:293–309
Ravindra K, Ameena M, Monika R, Kaushik A (2003) Seasonal variations in physico-chemical characteristics of river Yamuna in Haryana and its ecological best-designated use. J Environ Monit 5:419–426
Razmkhah H, Abrishamchi A, Torkian A (2010) Evaluation of spatial and temporal variation in water quality by pattern recognition techniques: a case study on Jajrood River (Tehran, Iran). J Environ Manag 91:852–860
Reza R, Singh G (2010) Heavy metal contamination and its indexing approach for river water. Int J Environ Sci Technol 7:785–792
Rout S, Behera AK, Patnaik A (2016) Water quality analysis of River Mahanadi in Sambalpur City. Int J Sci Res Publ 6(2):266–270
Samuel M, Taddese W, Richard D, Luc M (2007) Pollution status of Tinishu Akaki river and its tributaries (Ethiopia) evaluated using physico-chemical parameters, major ions, and nutrients. Bull Chem Soc Ethiop 21(1):13–22
Sin SN, Chua H, Lo W, Ng LM (2001) Assessment of heavy metal cations in sediments of Shing Mun River, Hong Kong. Environ Int 26:297–301
Singh KP, Malik A, Mohan D, Sinha S (2004) Multivariate statistical techniques for the evaluation of spatial and temporal variations in water quality of Gomti River (India): a case study. Water Res 38:3980–3992
Singh KP, Malik A, Sinha S (2005) Water quality assessment and apportionment of pollution sources of Gomti river (India) using multivariate statistical techniques: a case study. Anal Chim Acta 538:355–374
Steffens C, Klauck CR, Benvenuti T, Silva LB, Rodrigues MAS (2015) Water quality assessment of the Sinos River–RS, Brazil. Braz J Biol 75(4):62–67
Tesfamariam T (1989) Water pollution and natural resources degradation. A challenge to Ethiopia. In: Beyen D (ed) First natural resources conservation conference, 7–8 Feb 1989. IAR, Addis Ababa
Udebuana OO, Akaluka CK, Bashir KMI (2014) Assessment of physico-chemical parameters and water quality of surface water of Iguedo River, Ovia South-West Local Government, Edo State. J Nat Sci Res 4(24):12–20
U.S. Environmental Protection Agency (USEPA) (1999) Integrated risk information system (IRIS) on chromium VI. National Center for Environmental Assessment, Office of Research and Development, Washington
Varol M, Gokot B, Bekleyen A, Sen B (2011) Water quality assessment and apportionment of pollution sources of Tigris River (Turkey) using multivariate statistical techniques: A case study. River Res Appl. https://doi.org/10.1002/rra.1533
Vega M, Pardo R, Barrado E, Deban L (1998) Assessment of seasonal and polluting effects on the quality of river water by exploratory data analysis. Water Res 32:3581–3592
Yang H, Shen Z, Zhang J, Wang W (2007) Water quality characteristics along the course of the Huangpu River (China). J Environ Sci 19:1193–1998
Acknowledgements
The authors acknowledge the financial and material support made by Addis Ababa University, Center for Environmental Science and Wollega University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Eliku, T., Leta, S. Spatial and seasonal variation in physicochemical parameters and heavy metals in Awash River, Ethiopia. Appl Water Sci 8, 177 (2018). https://doi.org/10.1007/s13201-018-0803-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-018-0803-x