Abstract
Large cities face water quality and quantity problems due to increasing population and improper disposal of solid and liquid wastes. It is essential to monitor the water quality to take corrective measures. This study was carried out in one of the densely populated metropolitan cities in India to ascertain the suitability of groundwater for drinking and irrigation activity, identify the processes controlling the geochemistry of groundwater and the impact of Adyar River on the groundwater quality. Magnesium and pH concentration in groundwater of this area were within the maximum permissible limits of WHO standards. Sodium and potassium concentration of groundwater were greater than the permissible limit in 30.8 % and in 50 % of the samples, respectively. About 35 % of the groundwater samples were not permissible for drinking based on the electrical conductivity (EC). The EC of groundwater was increasing towards the coast. In general, the quality of groundwater for irrigation purpose vary from moderate to good based on Na%, magnesium hazard, residual sodium carbonate, sodium absorption ratio, permeability index, and USDA classification. Na–Cl and Ca–Mg–Cl were the dominant groundwater and surface water type. Increased ionic concentration of groundwater towards the eastern part of the study area is due to the discharge of industrial effluents and domestic sewage into the Adyar River. Seawater intrusion is also one of the reasons for Na–Cl dominant groundwater near the coast. Evaporation and ion exchange were the major processes controlling groundwater chemistry in this area. The groundwater quality of this region is affected by the contaminated surface water.
Similar content being viewed by others
Introduction
Several large or mega cities in some developing countries are not catered by 24 h of piped water supply. This necessitates people to depend on private wells to meet their daily needs. Further, indiscriminate disposal of wastes and letting domestic sewerage in storm water drains may result in contamination of groundwater. The problems of groundwater pollution are more in cities than in rural areas as the pollution load is higher because of the huge population.
Groundwater quality may be affected by natural factors, such as geology and geochemical processes. Geogenic sources are one of the cause for the variation in chemical composition of groundwater which changes with space and time (Madhavan and Subramanian 2007; Zahid et al. 2008; Vikas et al. 2009; Gunduz et al. 2009; Mamatha and Rao 2009; Brindha et al. 2011). It depends on the parent rock, intensity of weathering, residence time and external factors, such as precipitation, temperature, etc. Hydrogeochemical processes, such as, weathering, dissolution, mixing, ion exchange, etc. control the concentration of major and minor ions in groundwater (Rajmohan and Elango 2004; Liu et al. 2008; Singh et al. 2008; Rajmohan et al. 2009; Tirumalesh et al. 2010; Singh et al. 2011; Zhu and Schwartz 2011; Rajesh et al. 2012). The presence of pathogenic microorganisms in soil and groundwater may affect human, animal, and plant health (Schaffter and Parriaux 2002; Gallay et al. 2006; Collins et al. 2006; Abdelrahman and Eltahir 2010). Intense agriculture increases the risk of salinisation of the soil and groundwater. The use of fertilizers and pesticides also lead to pollution of groundwater which has been reported earlier (Singh and Sekhon 1979; Mahvi et al. 2005; Tagma et al. 2009; Peña-Haro et al. 2010). The risk of pollution due to effluents with complex composition exists in industrial areas. Heavy metals are often the common polluting component around these industrial sites (Rao 1993; Mondal et al. 2005; Gowd and Govil 2008; Shakeri et al. 2009; Brindha et al. 2010).
The chemical ions that are present in groundwater due to these reasons determine its suitability for drinking, agriculture, and industrial purposes. Assessment of water quality is of paramount importance; especially, in populated regions which depend on groundwater. Standards, such as World Health Organisation (WHO 1993), United States Environmental Protection Agency (USEPA 2003), Bureau of Indian Standards (BIS 2003), etc. help to ascertain the usability of water for various purposes. Assessment of groundwater quality in populated regions, including large cities based on drinking water standards have been carried out by several researchers (Sujatha and Reddy 2003; Howari et al. 2005; Rao et al. 2005; Raju 2007; Özcan et al. 2007; Umar et al. 2009; Gupta et al. 2009; Dar et al. 2011).
Chennai is one such metropolitan city in India and has several industrial areas in its outskirts. The residents of the city are provided with piped water supply only for a few hours in a day. For the rest of their needs, people depend on private wells as a source. Despite the underground sewage lines, untreated sewage is also let into open drains that may deteriorate the groundwater quality. Two major rivers in Chennai; namely, Adyar and Cooum rivers are heavily polluted due to the disposal of domestic sewage at several locations. The domestic sewage let out by community living on the banks of the river and also the partly or untreated sewerage from the neighborhood reach these surface water bodies. Few studies have been carried out in the past in certain locations of Chennai to ascertain the surface and groundwater quality. Giridharan et al. (2009, 2010) reported on the contamination of Cooum River by sewage. Adyar River water quality was also studied by Venugopal et al. (2009a). Gowri et al. (2008) reported the transport of ammonia, nitrate, phosphate, cadmium, lead, and zinc by Adyar and Cooum rivers as a result of land-based discharges estimated during low tide. Rajkumar et al. (2008) estimated the emission fluxes for Adyar River to be ≈2.5 × 108 g CH4/year and ≈2.4 × 106 g N2O/year. Similar to surface water quality several researchers reported on the groundwater quality also. In 1995, Ramesh et al. (1995a, b) studied the spatial changes in major and trace elements concentration in groundwater of Chennai. Somasundaram et al. (1993), Venugopal et al. (2008, 2009b) and Giridharan et al. (2008) studied the groundwater quality along Adyar and Cooum rivers and identified the various sources of pollution. Groundwater quality in major industrial zones of Chennai was studied by Kumaresan and Riyazuddin (2006). Contamination of groundwater by major ions and heavy metals around tanning industries located in parts of Chennai was reported by Kumar and Riyazuddin (2008), Brindha et al. (2010) and Brindha and Elango (2012). All these studies were helpful to some extent to understand the sources and the intensity of pollution.
The objective of this study is to assess the present quality of surface and groundwater, interaction between them and to determine the suitability of water for various purposes. The hydrochemical processes that control the chemistry of water in this area are also assessed. Government agencies have been attempting to restore the quality of the polluted rivers of Chennai. As a part of redeeming the quality of the Adyar River, Adyar Poonga (Adyar garden) has been constructed and the ecological restoration of the creek has been successfully completed in the beginning of 2011. Adyar and Cooum Rivers are to be cleaned and restored and already work in connection with this has commenced. Hence, this study will also serve as a background to access the improvement in surface water and groundwater quality in future.
Study area
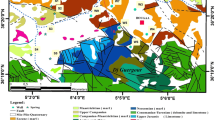
Chennai is the capital of Tamil Nadu and the fifth most populated city in India as per 2010 census. The study area which forms a part of Chennai is shown in Fig. 1. The metropolitan area covers 1,167 km2 with a population of 7.4 million people. Chennai experiences a tropical climate. The weather is hot most of the time in a year. From May to June, the temperature ranges from 38 °C to 42 °C and it varies from 18 °C to 32 °C during the months of December and January. The average annual rainfall is about 1,200 mm. Northeast (October to December) and southwest monsoon (July to September) contribute to 60 % and 40 % of annual rainfall. The city sometimes also receives rainfall when cyclones hit the Bay of Bengal.
Adyar River and Cooum River are the main waterways in the city. Three Lakes; namely, Red Hills, Sholavaram, and Chembarambakkam supply to the city’s water needs. These lakes of the city receive water mainly during the monsoons. Adyar River extends from Malaipattu tank, where the river starts in the west to Bay of Bengal in the east. An area of 80 km2 is considered in this study. The river flows in the South Chennai for nearly 50 km, and then enters the Bay of Bengal. The river receives water from Chembarambakkam Lake at Thiruneermalai. Thus, the Chembarambakkam Lake is considered as the main source of water to the Adyar River. This river is heavily polluted with effluents from domestic and industrial sources. Large quantity of sewage drains into the river as discussed earlier. Chembarambakkam Lake provides water supply to a part of the city, and it is one of the major sources of drinking water for the city.
Geology and hydrogeology
This region is mostly comprised of Archean crystalline rocks. These rocks occur as basement over the entire area and they outcrop in the western part of the region and also in the Adyar River bed. The Archean crystalline rocks include charnockites. These crystalline rocks are generally weathered in the top. The depth and intensity of weathering varies and in general its thickness varies from 4 to 15 m. The weathered rocks are overlaid by a thin soil cover and also alluvium especially along the river. Alluvium consists of sand, silt, and clay which occur in different proportion. The thickness of this soil or alluvium occurring above the basement crystalline rocks varies from 3 to 5 m. However, in the eastern part; especially, near the coast the thickness increases up to 25 m.
Groundwater occurs in this area under unconfined condition both in the upper soil/alluvium and the weathered/fractured crystalline rocks. The maximum depth of bore well in this region is about 100 m. Transmissivity in this area varies between 6 and 872 m2/d and the storativity varies between 2.9 × 10−4 and 4.5 × 10−3 (CGWB 2008). Maximum depth to groundwater table is about 28 m. The wells in the crystalline formation generally yield up to 7 lps (CGWB 2008).
Methodology
Data collection
Secondary data, such as rainfall, Chembarambakkam Lake water level, and groundwater level of few wells located nearer to the lake were collected from Public Works Department and Central Ground Water Board (CGWB), Chennai. This data were made use to interpret the long-term variation among these parameters, and to understand the relation between them.
Sampling and analytical methods
Groundwater samples were collected from 44 wells in February 2010 and from 34 wells in April 2010 (Fig. 1). Before the wells were chosen for collection of samples, a well-inventory survey was carried out and the electrical conductivity (EC) of nearly 60 wells were measured. Depending on the EC, among the wells located closely showing almost same EC, one representative well was selected. In addition, 21 surface water samples (Fig. 1) were collected (inclusive of Adyar River and Chembarambakkam Lake) during February 2010 and April 2010. Groundwater level was recorded using a water level meter (Solinst 100). EC and pH were measured in the field using Eutech portable digital meters. The pH meter was calibrated before use by 4.01, 7, and 10.01 buffer solution. EC meter was calibrated using 84 and 1,413 μS conductivity solution. Groundwater and surface water samples were collected in HDPE bottles of 500 ml capacity. These bottles were soaked in 1:1 dilute hydrochloric acid overnight and then washed three to four times with distilled water. Before the collection of samples, these bottles were rinsed with the sample. The bottles were labeled properly and were brought to the laboratory for analysis. Standard procedures were followed for the analysis of groundwater and surface water samples (APHA 1998). Calcium and magnesium were determined with 0.05 N EDTA solution titrimetrically. Carbonate and bicarbonate were estimated by titration with 0.01 N H2SO4. Flame photometer was used to measure the concentration of sodium and potassium. Chloride was determined by titrating against AgNO3. Sulphate in water samples was determined using spectrophotometer. The accuracy of analytical experiments was determined by calculating the ionic balance error, which was generally within ±5 %. Total dissolved solids (TDS) was calculated using the measured EC values by the relationship, TDS (mg/l) = EC (μS/cm) × 0.64 (Lloyd and Heathcote 1985). Total hardness (TH) was calculated by TH (mg/l) = 2.497Ca + 4.115Mg (Sawyer and McCarty 1978). Maps of the study area were prepared using Arc GIS 9.3 software. Surfer (version 8) was used for preparation of regional variation in groundwater level and statistical calculations were performed using Statistica.
Results and discussion
Surface water quality
It is essential to compare the chemical composition of surface and groundwater with standards, such as WHO and BIS to determine its usefulness. EC was high with 4,700 and 9,024 μS/cm in February and April 2010, respectively. Surface water is not being used for drinking purpose by the public and hence it has not been classified for ascertaining its suitability for drinking purpose. The pollution in the river could be understood from the brown to blackish gray colour of the samples collected. The increase in EC along the river flow is evident from Fig. 2. The mixing of domestic sewage in the river and dumping of solid waste along the river banks have heavily polluted the river. The river is presently used only for recreational purposes, such as boating. Thus, the water of Adyar River is suitable neither for drinking nor for irrigation.
Groundwater dynamics and quality
Groundwater flow
The groundwater flow is towards the east and it can be understood from the groundwater contour map shown in Fig. 3. As expected, the groundwater flow direction follows the topography of the area. The Adyar River mostly carries domestic wastewater and seawater near the coast. Hence, the water level in this river will be generally at mean sea level. When considering the river water level at mean sea level, it can be inferred that, in the upstream part, the groundwater is discharging into the river. However, in the eastern part, the river water will recharge the aquifer as the groundwater level is lower due to pumping.
Comparison of rainfall and water levels
Rainfall, lake water level and groundwater level of wells (Fig. 1) from January 2005 to December 2009 was compared to understand their relationship. As the rainfall increase, the lake and water level also increase during the subsequent months (Fig. 4). This shows that rainfall recharge has resulted in the increase in groundwater level after the monsoon. The variation is very clear with the increase in lake level and groundwater level in wells. In addition, the pattern of variation in rainfall and lake level is almost similar every year.
Drinking water quality
Although the people living in this area obtain piped water supplied by the Corporation of Chennai, this is limited for only few hours of the day and hence they also abstract groundwater by bore wells for daily use. It is essential that the water used for drinking and domestic purpose, such as cooking be free from colour, odor, turbidity, and toxic chemicals. To determine the suitability of water for such purposes, there are several standards laid by the National and International organizations. The groundwater in this region has been classified based on BIS (2003) and WHO (1993) standards to ascertain its suitability for drinking purposes (Table 1) based on pH and major ions.
pH and EC
pH in groundwater of the study area ranges from 6.5 to 8.1 with an average of 7.4, which indicates that groundwater of the study area is slightly alkaline in nature. pH was found to be within the permissible limit of 6.5–8.5 prescribed for drinking water by BIS (2003) and WHO (1993).
EC, a measure of the degree of the mineralization of the water ranges between 184 and 3,116 μS/cm at 25 °C with an average of 1,292 μS/cm. If water with high EC is consumed it may cause gastrointestinal irritation in human being (Singh et al. 2008). Hence, it is necessary that the EC which is dependent on the rock water interaction and thereby the residence time of the water in the rock (Eaton 1950) has to be within permissible limits. Groundwater was classified based on EC according to WHO standards (Table 2). Overall, 65 % of the groundwater samples are permissible for drinking purpose in the study area. There is only one sample which was harmful for human consumption. A higher EC may be attributed to anthropogenic activities prevailing in this area. The spatial variation in EC during February and April is shown in Fig. 5. Spatial variation in EC follows the groundwater flow direction (Figs. 3, 5). Groundwater is comparatively better (permissible for drinking) on the western part of the study area. High EC towards the east is due to accumulation and increase in the dissolved solids in the river, which recharges the groundwater.
Total dissolved solids
To ascertain the righteousness of groundwater for any purpose, it is essential to classify the groundwater depending upon TDS which is related to EC. Freeze and Cherry (1979) and Davis and DeWiest (1966) classification are available to assure the suitability of groundwater for drinking and irrigation activities. It seems from the Tables 3 and 4 that more than half, i.e., 68 % of groundwater is below 1,000 mg/l of TDS which is fresh water and permissible for drinking purpose without any health risk, while 32 % of the samples were brackish type as per Freeze and Cherry (1979) classification. According to Davis and DeWiest classification (1966), 32 % of the samples were suitable for irrigation activities.
Total hardness
Classification of water based on TH as suggested by Sawyer and McCarty (1978) is given in Table 5. Most of the samples were hard (48.7 %) with TH ranging between 150 and 300 mg/l. Few samples were very hard with TH above 300 (19.2 %). The long-term consumption of extremely hard water may result in increased incidence of urolithiasis, anencephaly, prenatal mortality, some types of cancer, and cardiovascular disorders (Durvey et al. 1991; Agrawal and Jagetia 1997). As per BIS (2003) standards, 80.8 % of the groundwater samples were desirable for drinking. Except for one sample, all the other samples were within the maximum permissible limit of 600 mg/l.
Major cations and anions
The concentration of various ions in the groundwater samples were compared with BIS and WHO standards which are given in Table 1. The minimum and maximum concentrations of calcium are 12 and 296 mg/l, respectively. The average concentration for calcium in groundwater is 60.3 mg/l. Although only 1.3 % of the samples were above the maximum permissible level of 200 mg/l, 23 % were above the desirable limit of 75 mg/l. Usually, calcium results in groundwater due to weathering from rocks and minerals. The concentration of magnesium in the study area ranges from 4.6 to 44.4 mg/l with an average of 19.8 mg/l. All the samples were within the desirable limit of 50 mg/l (WHO 1993) and maximum permissible limit of 150 mg/l (BIS 2003; WHO 1993). In addition, 84.6 % of them were within the BIS desirable limit of 30 mg/l. Concentration of sodium in groundwater ranged from 18.1 to 620 mg/l. Sodium beyond the maximum permissible limit of 200 mg/l was present in 30.8 % of groundwater samples. EURO Reports and Studies (1979) has documented that excessive salt intake seriously aggravates chronic congestive heart failure and ill effects due to high levels of sodium in drinking water. In addition, acute effects in humans, such as nausea, vomiting, convulsions, muscular twitching and rigidity, and cerebral and pulmonary edema may result due to higher levels of sodium intake (Department of National Health and Welfare 1992; Elton et al. 1963). Potassium concentration in groundwater ranges from 0.7 to 93.5 mg/l with an average value of 17.7 mg/l. 50 % of the samples were above the WHO prescribed maximum admissible limit of 12 mg/l. Normally adverse health effects due to ingestion of high concentration of potassium is rare in human beings. This is because potassium is rapidly excreted in the absence of pre-existing kidney damage and because large single doses usually induce vomiting (Gosselin et al. 1984).
The minimum, maximum, and mean values of bicarbonate in this study were found to be 36.6, 317.2, and 149.2 mg/l, respectively. Carbonate is absent in the groundwater of this area. High chloride may cause corrosion in metal pipes through which they are transported thereby increasing heavy metal content in the transported water and ultimately it reaches the drinking water system. Chloride concentration in groundwater vary from 35.5 to 1,223 mg/l. 1.3 % of groundwater samples had chloride above the maximum permissible limit of 1,000 mg/l, and 66.7 % of samples were within the permissible limit of 250 mg/l (BIS 2003). The desirable limit of chloride in drinking water as per WHO standards is 200 mg/l, and 51.3 % of the groundwater samples were within this limit. The concentration of sulphate varied between a minimum of 3.1 mg/l to a maximum of 583.9 mg/l with an average of 141.5 mg/l. 75.6 % of the samples were within the desirable limit of 200 mg/l (WHO 1993), whereas 3.8 % were above the maximum permissible limit of 400 mg/l (BIS 2003; WHO 1993). Maiti (1982) and Rao (1993) reported that high concentration of sulphate in drinking water may create respiratory problems in humans. With respect to major ions, the groundwater is suitable for domestic purpose except for the presence of excess sodium and potassium in some locations. However, the groundwater is likely to be contaminated by microbes due to mixing of wastewater.
Irrigation water quality
The groundwater in the study area is being used for agriculture purposes in the western outskirts of the city, as the surface water resources are polluted. Water used for irrigation should meet the requirements for crop growth to achieve maximum crop productivity. EC and sodium play a vital role in suitability of water for irrigation. Several methods are available to ensure the suitability of the water used for irrigation purpose, such as magnesium hazard (MH), residual sodium carbonate (RSC), sodium absorption ratio (SAR), permeability index (PI), and United States Department of Agriculture (USDA) classification.
If EC of irrigated water is high, it will affect root zone and water flow. A guideline has been established by USDA Salinity Laboratory as given in Freeze and Cherry (1979) to determine the suitability of water for irrigation based on EC. Table 6 indicates that only 7.7 % of the samples were not found suitable for irrigation.
Soil containing large proportions of sodium with carbonate as the predominant anions is termed as alkali soil, whereas with chloride or sulphate as the predominant cations is termed as saline soil. Both the soil types will not support plant growth. Thus, sodium is an important parameter for irrigation waters. It is denoted as sodium percentage or percent sodium (Na%). It is calculated from the formula given below (Wilcox 1955), where all concentrations are expressed in meq/l.
The suitability of water for irrigation based on Na% given in Table 7 shows that 52.6 % of the samples are doubtful while 6.4 % are unsuitable. Groundwater samples of the study area are plotted in the Wilcox’s diagram (Wilcox 1955) to classify the water for irrigation, wherein EC is plotted against Na%. Figure 6 shows that 35.9 % of the groundwater samples are good to permissible for agriculture while the rest of them are doubtful to unsuitable.
RSC which is frequently used to determine irrigation water quality is computed, using the following formula where ions are expressed in meq/l.
If the concentration of carbonate and bicarbonate is in excess than the concentration of calcium and magnesium, it will be a problem to the soil fertility and growth of plants. Most of the samples (96.2 %) (Table 8) were within the safe category for irrigation on the basis of RSC.
One of the most important parameter for the determination of desirability of irrigation water is SAR. It is calculated by (Richards 1954),
where all the concentration is in meq/l. Groundwater collected from the study area comes under excellent to good category based on SAR values (Table 9). In the United States Salinity Laboratory (USSL) diagram proposed by Richards (1954), water used for irrigation can be classified into four types—C1, C2, C3, and C4 based on salinity hazard and S1, S2, S3, and S4 based on sodium hazard. Figure 7 shows all the groundwater samples plotted on the USSL diagram. Most of the samples (37.2 %) fall under C2S1 type. 29.5 % were C3S1 and 25.6 % were C3S2 types, respectively. Few samples also belonged to C1S1 (1.3 %), C2S2 (3.8 %), and C3S3 (2.6 %). Overall, only 33 groundwater samples in this area are suitable for irrigation based on the salinity hazard and sodium hazard.
Magnesium hazard denoted by MH, calculated using the formula,
where the concentrations are in meq/l (Szabolcs and Darab 1964). Magnesium hazard above 50 meq/l is considered to be unsuitable for irrigation. A comparatively smaller percentage (16.7 %) was not fit for irrigation, whereas 65 samples were good for irrigation.
The suitability of groundwater for irrigation was also determined based on calcium, magnesium, sodium, and bicarbonate ions. This is given by PI which was calculated using the equation,
where concentrations are in meq/l. Doneen (1964) put forth that Class I and II waters are considered to be good and suitable for irrigation, while class III water is unsuitable for irrigation. Seven groundwater samples (9 %) were not suitable for irrigation, whereas rest of the samples is good (Fig. 8).
Physical and geochemical processes
Geochemical processes are important as they decide on the composition of groundwater and are the cause for spatial and temporal variation in groundwater quality. The different kinds of processes that occur depend on the nature of aquifer material. Usually, groundwater chemistry in any region is heterogeneous as a result of diverse sources and geochemical processes. The various geochemical processes that control the groundwater chemistry of this region are identified and given below.
Evaporation
Evaporation is an important process in the study area which is understood from the plot between Na versus Cl (Fig. 9). This figure shows that most of samples plot around the fresh water evaporation line, emphasizing that evaporation plays a major role in deciding the chemical composition of groundwater in this area.
Ion exchange
Chloro-alkaline indices I and II (CAI-I and CAI-2) proposed by Schoeller (1965) help in determining the ion exchange process in groundwater. It is calculated using the formulae:
(all values are measured in meq/l).
When there is an exchange between sodium or potassium in groundwater with calcium or magnesium in the aquifer material, CAI I and II are positive and it indicates reverse ion exchange. During ion exchange process, there is exchange between calcium or magnesium in groundwater with sodium or potassium in the formation. In this case both the indices are negative. Figure 10 which shows the CAI I and II of the groundwater in this region indicate that reverse ion exchange is the dominant process. There are few wells which undergo ion exchange also.
If there occurs reverse ion exchange, the relation between Ca + Mg and SO4 + HCO3 will be close to 1:1 equiline denoting dissolution of calcite, dolomite, and gypsum (Fig. 11a). Reverse ion exchange can also be identified by the relationship between Na–Cl and Ca + Mg–HCO3–SO4. Fisher and Mullican (1997) put forth that such a relationship will be linear with a slope of −1. Groundwater samples of this study plot in a linear fashion (Fig. 11b) and the slope is −0.75. It is hence apparent that reverse ion exchange is one of the important processes controlling the groundwater chemistry of this area.
Surface water and groundwater interaction
The dominance of the major ions is as Na+ > Ca2+ > K+ > Mg2+ for cations and Cl− > HCO3− > SO42− > CO32− for anions. The order of ions was same for groundwater as well as surface water. For the geochemical classification of groundwater and surface water and interpretation of chemical data, Chadha (1999) diagram was used. This is a modified form of Piper trilinear diagram (Piper 1944) in which the major cations and anions are plotted in a rectangular plot. It is a simpler way of identifying the water type as compared to Piper diagram, and it does not require any special software other than a spreadsheet. The Chadha diagram is plotted in the following way: the difference in milliequivalent percentage between alkaline earths (Ca + Mg) and alkali metals (Na + K) expressed as percentage is plotted on the x axis. The difference in milliequivalent percentage between weak acidic anions (CO3 + HCO3) and strong acidic anions (Cl + SO4) is plotted on the y axis. The rectangular field resulting from this is similar to the diamond shaped field in the Piper diagram and describes the overall character of the water. Thus, there are eight fields in the rectangular plot which represent eight different water types as in case of Piper diagram. Those eight water types (Fig. 12a) are (1) alkaline earths exceed alkali metals, (2) alkali metals exceed alkaline earths, (3) weak acidic anions exceed strong acidic anions, (4) strong acidic anions exceed weak acidic anions, (5) alkaline earths and weak acidic anions exceed both alkali metals and strong acidic anions, respectively (such water has temporary hardness), (6) alkaline earths exceed alkali metals and strong acidic anions exceed weak acidic anions (such water has permanent hardness), (7) alkali metals exceed alkaline earths and strong acidic anions exceed weak acidic anions, and (8) alkali metals exceed alkaline earths and weak acidic anions exceed strong acidic anions (Chadha 1999).
Groundwater and surface water samples plotted in the Chadha diagram to know the water type is shown in Fig. 12a. Na–Cl was the dominant groundwater type with 71 % of the samples being this type. The second dominant groundwater type was Ca–Mg–Cl. It was the same case with surface water also with Na–Cl being the first dominant and Ca–Mg–Cl being the second dominant water type. This shows the groundwater and surface water are of the same type chemical composition in this study area. The dominance of Na–Cl water type is due to seawater intrusion, as well as recharge of saline water from the river. Figure 12b–e shows that both groundwater and surface water have the same chemical ratios indicating the interaction between the river water and groundwater is playing a major role.
To confirm whether the groundwater quality is influenced by the surface water running in this region, Scholler diagram was plotted between nearby surface water and groundwater samples which is shown in Fig. 13. This semi-logarithmic diagram represents concentration of major ions in meq/l. Surface water and groundwater concentration of major ions plotted (Fig. 13) show that wherever there is rise in ion concentration in surface water, there is also rise in concentration of that particular ion in groundwater and wherever there is fall in ion concentration in surface water there is also fall in concentration of that particular ion in groundwater. The groundwater and surface water characteristics in the area are similar as they show same type of variation in major ion concentration. This implies that the surface water has an influence on groundwater quality. It is noticed that there are inputs of untreated/partially treated/treated industrial waste and domestic sewage at few points of the Adyar River. This might be the major source polluting the surface water which has apparently a strong influence on the groundwater quality that is supported by Fig. 13. The similarity in the percentage of major ions between the river water and groundwater indicate that they are interrelated. During monsoonal rains, the river flow will result in recharging of the groundwater zone and the river stage will be more than the groundwater table. However, in other periods, groundwater will be discharged into the river; especially, in the western part of the area.
Limitations
This study concentrates mainly on the major ion chemistry in this area. As the sources are diverse from domestic sewage and industrial effluents, it is essential to carry out trace metal and microbial analysis in this region.
Conclusion
Groundwater and surface water interaction was studied in Chennai city, India. Suitability of groundwater for drinking and irrigation activity was assessed and the geochemical processes controlling the groundwater quality were identified. There was wide variation in groundwater and surface water quality with respect to drinking and irrigation water standards. Na–Cl type water was dominant in surface and groundwater. EC of groundwater increased towards the east following the general groundwater flow direction. Evaporation and ion exchange are the dominant processes controlling the groundwater chemical composition. Surface and groundwater samples showed a similar trend in the composition of ions. The surface water which is contaminated by partly or untreated domestic sewage has penetrated through the soil and contaminated the groundwater of this region. To improve the river water quality ecological restoration is planned to be implemented shortly by the Government agencies. Continuous monitoring of water quality in this area will help in understanding the progressive improvement in groundwater and surface water quality during the process of restoration.
References
Abdelrahman AA, Eltahir YM (2010) Bacteriological quality of drinking water in Nyala, South Darfur Sudan. Environ Monit Assess 175(1–4):37–43
Agrawal V, Jagetia M (1997) Hydrogeochemical assessment of groundwater quality in Udaipur city, Rajasthan, India. In: Proceedings of National conference on dimension of environmental stress in India, Department of Geology, MS University, Baroda, India, 151–154
APHA (American Public Health Association) (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association/American Water Works Association/Water Environment Federation, Washington DC
BIS (Bureau of Indian Standards Specification for drinking water) (2003) IS:10500:91. Revised 2003, Bureau of Indian Standards, New Delhi
Brindha K, Elango L, Rajesh VG (2010) Occurrence of chromium and copper in groundwater around tanneries in Chromepet area of Tamil Nadu, India. Indian J Environ Prot 30(10):818–822
Brindha K, Rajesh R, Murugan R, Elango L (2011) Fluoride contamination in groundwater in parts of Nalgonda district, Andhra Pradesh, India. Environ Monit Assess 172:481–492
Brindha K, Elango L (2012) Impact of tanning industries on groundwater quality near a metropolitan city in India. Water Resour Manag 26(6):1747–1761
CGWB (Central Ground Water Board) (2008) Central Ground Water Board’s District groundwater brochure. Chennai district, Tamil Nadu, p 19
Chadha DK (1999) A proposed new diagram for geochemical classification of natural water and interpretation of chemical data. Hydrogeol J 7:431–439
Collins KE, Cronin AA, Rueedi J, Pedley S, Joyce E, Humble PJ, Tellam JH (2006) Fate and transport of bacteriophage in UK aquifers as surrogates for pathogenic viruses. Eng Geol 85(1–2):33–38
Dar IA, Sankar K, Dar MA (2011) Spatial assessment of groundwater quality in Mamundiyar basin, Tamil Nadu, India. Environ Monit Assess 178:437–447
Davis SN, DeWiest RJM (1966) Hydrogeology. Wiley, NewYork
Department of National Health and Welfare (1992) Guidelines for Canadian drinking water quality, Supporting documentation, Ottawa, Canada
Doneen LD (1964) Water quality for Agriculture. Department of Irrigation, University of California, Davis, p 48
Durvey VS, Sharma LL, Saini VP, Sharma BK (1991) Handbook on the methodology of water quality assessment. Rajasthan Agriculture University, India
Eaton EM (1950) Significance of carbonates in irrigation waters. Soil Sci 69:123–133
Elton NW, Elton WJ, Narzareno JP (1963) Pathology of acute salt poisoning in infants. Am J Clin Pathol 39:252–264
EURO Reports and Studies (1979) EURO Reports and Studies No. 2. Sodium, chlorides and conductivity in drinking water. Copenhagen, WHO Regional Office for Europe
Fisher SR, Mullican WF (1997) Hydrogeochemical evolution of sodium-sulphate and sodium-chloride groundwater beneath the northern Chihuahua desert, Trans-Pecos, Texas, USA. Hydrogeol J 5:4–16
Freeze RA, Cherry JA (1979) Groundwater. Prentice Hall Inc., New Jersey, p 604
Gallay A, De Valk H, Cournot M, Ladeuil B, Hemery C, Castor C, Bon F, Mégraud F, Le Cann P, Desenclos JC (2006) A large multi-pathogen waterborne community outbreak linked to faecal contamination of a groundwater system, France. Clin Microbiol Infect 12(6):561–570
Giridharan L, Venugopal T, Jayaprakash M (2008) Evaluation of the seasonal variation on the geochemical parameters and quality assessment of the groundwater in the proximity of river Cooum, Chennai, India. Environ Monit Assess 143:161–178
Giridharan L, Venugopal T, Jayaprakash M (2009) Assessment of water quality using chemometric tools: a case study of river Cooum, South India. Arch Environ Contam Toxicol 56:654–669
Giridharan L, Venugopal T, Jayaprakash M (2010) Identification and evaluation of hydrogeochemical processes on river Cooum, south India. Environ Monit Assess 162:277–289
Gosselin RE, Smith RP, Hodge HC (1984) Clinical toxicology of commercial products, 5th edn. Williams & Wilkins, Baltimore
Gowd SS, Govil PK (2008) Distriburtion of heavy metals in surface water of Ranipet industrial area in Tamil Nadu, India. Environ Monit Assess 136:197–207
Gowri VS, Ramachandran S, Ramesh R, Pramiladevi IRR, Krishnaveni K (2008) Application of GIS in the study of mass transport of pollutants by Adyar and Cooum Rivers in Chennai, Tamilnadu. Environ Monit Assess 138:41–49
Gunduz O, Simsek C, Hasozbek A (2009) Arsenic pollution in the groundwater of Simav Plain, Turkey: its impact on water quality and human health. Water, Air and Soil Pollut 205(1–4):43–62
Gupta S, Dandele PS, Verma MB, Maithani PB (2009) Geochemical assessment of groundwater around Macherla-Karempudi Area, Guntur District, Andhra Pradesh. J Geol Soc India 73:202–212
Howari FM, Abu-Rukah Y, Shinaq R (2005) Hydrochemical analyses and evaluation of groundwater resources of North Jordan. Water Resour 32(5):555–564
Kumar AR, Riyazuddin P (2008) Application of chemometric techniques in the assessment of groundwater pollution in a suburban area of Chennai city, India. Curr Sci 94(8):1012–1022
Kumaresan M, Riyazuddin P (2006) Major ion chemistry of environmental samples around sub-urban of Chennai city. Curr Sci 91(12):1668–1677
Liu CW, Jang CS, Chen CP, Lin CN, Lou KL (2008) Characterization of groundwater quality in Kinmen Island using multivariate analysis and geochemical modelling. Hydrol Processes 22:376–383
Lloyd JW, Heathcote JA (1985) Natural inorganic hydrochemistry in relation to groundwater. Clarendon Press, Oxford
Madhavan N, Subramanian V (2007) Environmental impact assessment, remediation and evolution of fluoride and arsenic contamination process in groundwater. Groundwater 128–155
Mahvi AH, Nouri J, Babaei AA, Nabizadeh R (2005) Agricultural activities impact on groundwater nitrate pollution. Int J Environ Sci Technol 2(1):41–47
Maiti TC (1982) The dangerous acid rain. Sci Report 9:360–363
Mamatha P, Rao SM (2009) Geochemistry of fluoride rich groundwater in Kolar and Tumkur Districts of Karnataka. Environ Earth Sci 61(1):131–142
Mondal NC, Saxena VK, Singh VS (2005) Assessment of groundwater pollution due to tannery industries in and around Dindigul, Tamilnadu, India. Environ Geol 48:149–157
Özcan H, Ekinci H, Baba A, Kavdır Y, Yüksel O, Yi˘gini Y (2007) Assessment of the water quality of Troia for the multipurpose usages. Environ Monit Assess 130:389–402
Peña-Haro S, Llopis-Albert C, Pulido-Velazquez M, Pulido-Velazquez D (2010) Fertilizer standards for controlling groundwater nitrate pollution from agriculture: El Salobral-Los Llanos case study Spain. J Hydrol 392(3–4):174–187
Piper AM (1944) A graphical procedure in the geochemical interpretation of water analysis. Trans Am Geophys Union 25:914–928
Rajesh R, Brindha K, Murugan R, Elango L (2012) Influence of hydrogeochemical processes on temporal changes in groundwater quality in a part of Nalgonda district, Andhra Pradesh India. Environ Earth Sci 65:1203–1213
Rajkumar AN, Barnes J, Ramesh R, Purvaja R, Upstill-Goddard RC (2008) Methane and nitrous oxide fluxes in the polluted Adyar River and estuary SE India. Marine Pollut Bull 56:2043–2051
Rajmohan N, Elango L (2004) Identification and evolution of hydrogeochemical processes in the groundwater environment in an area of the Palar and Cheyyar River Basins, Southern India. Environ Geol 46(1):47–61
Rajmohan N, Al-Futaisi A, Al-Touq S (2009) Geochemical process regulating groundwater quality in a coastal region with complex contamination sources: Barka, Sultanate of Oman. Environ Earth Sci 59(2):385–398
Raju NJ (2007) Hydrogeochemical parameters for assessment of groundwater quality in the upper Gunjanaeru River basin, Cuddapah District, Andhra Pradesh, South India. Environ Geol 52:1067–1074
Ramesh R, Kumar KS, Eswaramoorthi S, Purvaja GR (1995a) Migration and contamination of major and trace elements in groundwater of Madras City, India. Environ Geol 25:126–136
Ramesh R, Purvaja GR, Ika RV (1995b) The problem of groundwater pollution: a case study from Madras city, India. Man’s influence on freshwater ecosystems and water use (Proceedings of a Boulder Symposium, July 1995). IAHS Publ. no. 230, 147–157
Rao NS (1993) Environmental impact of industrial effluents in groundwater regions of Visakhapatnam Industrial Complex. Indian J Geol 65:35–43
Rao NS, Nirmala IS, Suryanarayana K (2005) Groundwater quality in a coastal area: a case study from Andhra Pradesh, India. Environ Geol 48(4–5):543–550
Richards LA (1954) Diagnosis and improvement of saline and Alkali soils. USDA handbook, 60, pp 160
Sawyer CN, McCarty PL (1978) Chemistry of environmental engineering, 3rd ed. Series in water resources and environmental engineering, McGraw–Hill, New York; pp 532
Schaffter N, Parriaux A (2002) Pathogenic-bacterial water contamination in mountainous catchments. Water Res 36(1):131–139
Schoeller H (1965) Qualitative evaluation of groundwater resources. In: methods and techniques of groundwater investigations and development, UNESCO, 99 54–63
Shakeri A, Moore F, Mohammadi Z, Raeisi E (2009) Heavy metal contamination in the Shiraz industrial complex zone groundwater, South Shiraz, Iran. World Appl Sci J 7(4):522–530
Singh AK, Mondal GC, Kumar S, Singh TB, Tewary BK, Sinha A (2008) Major ion chemistry, weathering processes and water quality assessment in upper catchment of Damodar River basin, India. Environ Geol 54:745–758
Singh B, Sekhon GS (1979) Nitrate pollution of groundwater from farm use of nitrogen fertilizers: a review. Agric Environ 4(3):207–225
Singh K, Hundal HS, Singh D (2011) Geochemistry and assessment of hydrogeochemical processes in groundwater in the southern part of Bathinda district of Punjab, northwest India. Environ Earth Sci. doi:10.1007/s12665-011-0989-9
Somasundaram MV, Ravindran G, Tellam JH (1993) Ground-water pollution of the Madras Urban aquifer, India. Ground Water 31:4–11
Sujatha D, Reddy BR (2003) Quality characterization of groundwater in the south-eastern part of the Ranga Reddy district, Andhra Pradesh, India. Environ Geol 44:579–586
Szabolcs I, Darab C (1964) The influence of irrigation water of high sodium carbonate content of soils, Proceedings of 8th ISSS, Trans vol. II, 802–812
Tagma T, Hsissou Y, Bouchaou L, Bouragba L, Boutaleb S (2009) Groundwater nitrate pollution in Souss-Massa basin (south-west Morocco). Afr J Environ Sci Technol 3(10):301–309
Tirumalesh K, Shivanna K, Sriraman AK, Tyagi AK (2010) Assessment of quality and geochemical processes occurring in groundwaters near central air conditioning plant site in Trombay, Maharashtra, India. Environ Monit Assess 163(1–4):171–184
Umar R, Ahmed I, Alam F, Khan MM (2009) Hydrochemical characteristics and seasonal variations in groundwater quality of an alluvial aquifer in parts of Central Ganga Plain, Western Uttar Pradesh, India. Environ Geol 58:1295–1300
USEPA (United States Environmental Protection Agency) (2003) Current drinking water standards. Ground water and drinking water protection agency: report prepared by Wade Miller Associates, pp 12
Venugopal T, Giridharan L, Jayaprakash M (2008) Groundwater quality assessment using chemometric analysis in the Adyar river, south India. Arch Environ Contam Toxicol 55:180–190
Venugopal T, Giridharan L, Jayaprakash M, Periakali P (2009a) Environmental impact assessment and seasonal variation study of the groundwater in the vicinity of river Adyar, Chennai, India. Environ Monit Assess 149:81–97
Venugopal T, Giridharan L, Jayaprakash M, Velmurugan PM (2009b) A comprehensive geochemical evaluation of the water quality of river Adyar, India. Bull Environ Contam Toxicol 82:211–217
Vikas C, Kushwaha RK, Pandit MK (2009) Hydrochemical status of groundwater in district Ajmer (NW India) with reference to fluoride distribution. J Geol Soc India 73:773–784
WHO (World Health Organisation) (1993) Guidelines for drinking water quality, vol. 1, 2nd edn, Recommendations, WHO, Geneva, pp 130
Wilcox LV (1955) Classification and use of irrigation waters, USDA, circular 969, Washington DC, USA
Zahid A, Hassan MQ, Balke KD, Flegr M, Clark DW (2008) Groundwater chemistry and occurrence of arsenic in the Meghna floodplain aquifer, southeastern Bangladesh. Environ Geol 54(6):1247–1260
Zhu C, Schwartz W (2011) Hydrogeochemical processes and controls on water quality and water management. Elements 7(3):169–174
Acknowledgments
The authors would like to thank the Department of Science and Technology’s Funds for Improvement in Science and Technology scheme (Grant No. SR/FST/ESI-106/2010), University Grants Commission’s Special Assistance Programme [Grant No. UGC DRS II F.550/10/DRS/2007(SAP-1)] and University Grants Commission’s Centre with Potential for Excellence in Environmental Science [Grant No. F.No.1-9/2002 (NS/PE)] for their financial support which helped in creating facilities to carry out part of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under license to BioMed Central Ltd. Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Brindha, K., Neena Vaman, K.V., Srinivasan, K. et al. Identification of surface water-groundwater interaction by hydrogeochemical indicators and assessing its suitability for drinking and irrigational purposes in Chennai, Southern India. Appl Water Sci 4, 159–174 (2014). https://doi.org/10.1007/s13201-013-0138-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-013-0138-6