Abstract
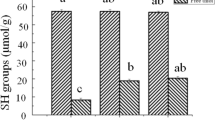
Salting process is widely used in the process of meat products, whereas few studies have revealed the digestibility of actomyosin after salting treatment, which is closely related with the nutrition of meat. This work reported effect of salting on the structural change and digestibility of actomyosin before and after heat treatment. Actomyosin in 0.4 M and 0.8 M of NaCl had higher content of disulfide bonds, and actomyosin in 0.4 M NaCl showed the largest particle sizes before and after heat treatment. In addition, actomyosin in 0.6 M and 0.8 M of NaCl was oxidized more severely after heat treatment. Based on peptidomics analysis by using liquid chromatography tandem mass spectrometry (LC–MS/MS), actomyosin in 0.6 M was digested more easily, which was followed by sample in 0.8 M and 0.4 M of NaCl in descending order. The lowest digestibility of actomyosin in 0.4 M NaCl was related with its higher content of disulfide bond and severer aggregation behavior. The lower digestibility of actomyosin in 0.8 M NaCl should be related with the higher content of disulfide bonds and surface oxidation. These results highlight the crucial role of salting process in affecting the digestibility of meat protein.






Similar content being viewed by others
References
Aliño M, Grau R, Fernández-Sánchez A, Arnold A, Barat JM (2010) Influence of brine concentration on swelling pressure of pork meat throughout salting. Meat Sci 86:600–606
Bax ML, Aubry L, Ferreira C, Daudin JD, Gatellier P, Rémond D, Santé-Lhoutellier V (2012) Cooking temperature is a key determinant of in vitro meat protein digestion rate: investigation of underlying mechanisms. J Agric Food Chem 60:2569–2576
Berhe DT, Engelsen SB, Hviid MS, Lametsch R (2014) Raman spectroscopic study of effect of the cooking temperature and time on meat proteins. Food Res Int 66:123–131
Beveridge T, Toma SJ, Nakai S (1974) Determination of SH-and SS-groups in some food proteins using Ellman’s reagent. J Food Sci 39:49–51
Brisson G, Britten M, Pouliot Y (2007) Heat-induced aggregation of bovine lactoferrin at neutral pH: effect of iron saturation. Int Dairy J 17:617–624
Cao Y, Xia T, Zhou G, Xu X (2012) The mechanism of high pressure-induced gels of rabbit myosin. Innov Food Sci Emerg 16:41–46
Chen X, Tume RK, Xu XL, Zhou GH (2015) Solubilization of myofibrillar proteins in water or low ionic strength media: classical techniques, basic principles and novel functionalities. Crit Rev Food Sci 57:3260–3280
Debiemme-Chouvy C, Haskouri S, Cachet H (2007) Study by XPS of the chlorination of proteins aggregated onto tin dioxide during electrochemical production of hypochlorous acid. Appl Surf Sci 253:5506–5510
Du XJ, Sun YY, Pan DD, Wang Y, Ou CR, Cao JX (2018a) The effect of structural change on the digestibility of sarcoplasmic proteins in Nanjing dry-cured duck during processing. Poultry Sci 97:4450–4457
Du XJ, Sun YY, Pan DD, Wang Y, Ou CR, Cao JX (2018b) Change of the structure and the digestibility of myofibrillar proteins in Nanjing dry-cured duck during processing. J Sci Food Agric 98:3140–3147
Dunn BM (2001) Overview of pepsin-like aspartic peptidases. Curr Protoc Protein Sci 25:1–21
Fox PF, Walley BF (1971) Influence of sodium chloride on the proteolysis of casein by rennet and by pepsin. J Dairy Res 38:165–170
Gilani GS, Xiao CW, Cockell KA (2012) Impact of antinutritional factors in food proteins on the digestibility of protein and the bioavailability of amino acids and on protein quality. Brit J Nutr 108:S315–S332
Guo X, Yao H, Chen Z (2007) Effect of heat, rutin and disulfide bond reduction on in vitro pepsin digestibility of Chinese tartary buckwheat protein fractions. Food Chem 102:118–122
Hamaker BR, Kirleis AW, Butler LG, Axtell JD, Mertz ET (1987) Improving the in vitro protein digestibility of sorghum with reducing agents. Proc Natl Acad Sci USA 84:626–628
Hu L, Ren S, Shen Q, Ye X, Chen J, Ling J (2018) Protein oxidation and proteolysis during roasting and in vitro digestion of fish (Acipenser gueldenstaedtii). J Sci Food Agric 98:5344–5351
Kumar P, Chatli MK, Verma AK, Mehta N, Malav OP, Kumar D (2015) Sharma N Quality, functionality and shelf life of fermented meat and meat products. A review. Crit Rev Food 57:2844–2856
Li L, Liu Y, Zou X, He J, Xu X, Zhou G, Li C (2017) In vitro protein digestibility of pork products is affected by the method of processing. Food Res Int 92:88–94
Li-Chan ECY (1996) The applications of Raman spectroscopy in food science. Trends Food Sci Technol 11:361–370
Liu R, Zhao SM, Xiong SB, Qiu CG, Xie BJ (2008) Rheological properties of fish actomyosin and pork actomyosin solutions. J Food Eng 85:173–179
Montel MC, Reitz J, Talon R, Berdague JL, Roussetakrim S (1996) Biochemical activities of micrococcaceae and their effects on the aromatic profiles and odours of a dry sausage model. Food Microbiol 13:489–499
Nayak R, Kenney PB, Slider S (1996) Protein extractability of turkey breast and thigh muscle with varying sodium chloride solutions as affected by calcium, magnesium and zinc chloride. J Food Sci 61:1149–1154
Nguyen MV, Thorarinsdottir KA, Gudmundsdottir A, Thorkelsson G, Arason S (2011) The effects of salt concentration on conformational changes in cod (Gadus morhua) proteins during brine salting. Food Chem 125:1013–1019
Offer G, Trinick J (1983) On the mechanism of water holding in meat: the swelling and shrinking of myofibrils. Meat Sci 8:245–281
Promeyrat A, Daudin JD, Gatellier P (2013) Kinetics of protein physicochemical changes induced by heating in meat using mimetic models: (1) Relative effects of heat and oxidants. Food Chem 138:581–589
Sharedeh D, Gatellier P, Astruc T, Daudin JD (2015) Effects of pH and NaCl levels in a beef marinade on physicochemical states of lipids and proteins and on tissue microstructure. Meat Sci 110:24–31
Si JL, Zheng JQ, Li H, Zhang YL (2015) Effect of salt content on the denaturation of pike eel (Muraenesox cinereus Forsskål, 1775) actomyosin. J Appl Ichthyol 31:767–770
Soladoye OP, Juárez ML, Aalhus JL, Shand P, Estévez M (2015) Protein oxidation in processed meat: mechanisms and potential implications on human health. Compr Rev Food Sci Food Saf 14:106–122
Sun WZ, Li QY, Zhou FB, Zhao HF, Zhao MM (2014) Surface characterization of oxidized myofibrils using X-ray photoelectron spectroscopy and scanning electron microscopy. J Agric Food Chem 62:7507–7514
Thorarinsdottir KA, Arason S, Geirsdottir M, Bogason SG, Kristbergsson K (2002) Changes in myofibrillar proteins during processing of salted cod (Gadus morhua) as determined by electrophoresis and differential scanning calorimetry. Food Chem 77:377–385
Thorarinsdottir KA, Arason S, Sigurgisladottir S, Valsdottir T, Tornberg E (2011) Effects of different pre-salting methods on protein aggregation during heavy salting of cod fillets. Food Chem 124:7–14
Zhao D, Li L, Le TT, Larsen LB, Su G, Liang Y, Li B (2017) Digestibility of glyoxal-glycated β-casein and β-lactoglobulin and distribution of peptide-bound advanced glycation end products in gastrointestinal digests. J Agric Food Chem 65:5778–5788
Zhao D, Li L, Xu D, Sheng B, Qin D, Chen J, Li B, Zhang X (2018) Application of ultrasound pretreatment and glycation in regulating the heat-induced amyloid-like aggregation of β-lactoglobulin. Food Hydrocoll 80:122–129
Zhao D, He J, Zou X, Xie Y, Xu X, Zhou G, Li C (2019) Influence of hydrothermal treatment on the structural and digestive changes of actomyosin. J Sci Food Agric 99:6209–6218
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31530054), the modern agricultural industry technology system (CARS35), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PADP) and Overseas Expertise Introduction Center for Discipline Innovation (“111 Center”) On Quality & Safety Control and Nutrition of Muscle Food.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, D., He, J., Zou, X. et al. Influence of salting process on the structure and in vitro digestibility of actomyosin. J Food Sci Technol 57, 1763–1773 (2020). https://doi.org/10.1007/s13197-019-04210-w
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-019-04210-w