Abstract
Introduction
Cannabis’ effect on seizure activity is an emerging topic that remains without consensus and merits further investigation. We therefore performed a scoping review to identify the available evidence and knowledge gaps within the existing literature on cannabis product exposures as a potential cause of seizures in humans.
Methods
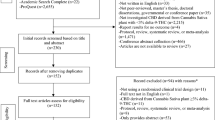
A scoping review was conducted in accordance with the PRISMA Extension for Scoping Reviews guidelines. The PubMed and Scopus databases were searched over a 20-year period from the date of the database query (12/21/2020). Inclusion criteria were (1) English language original research articles, (2) inclusion of human subjects, and (3) either investigation of seizures as a part of recreational cannabinoid use OR of exogenous cannabinoids as a cause of seizures.
Results
A total of 3104 unique articles were screened, of which 68 underwent full-text review, and 13 met inclusion/exclusion criteria. Ten of 11 studies evaluating acute cannabis exposures reported a higher seizure incidence than would be expected based on the prevalence of epilepsy in the general and pediatric populations (range 0.7–1.2% and 0.3–0.5% respectively). The remaining two studies demonstrated increased seizure frequency and/or seizure-related hospitalization in recreational cannabis users and those with cannabis use disorder.
Conclusions
This scoping review demonstrates that a body of literature describing seizures in the setting of cannabis exposure exists, but it has several limitations. Ten identified studies showed a higher than expected incidence of seizures in populations exposed to cannabis products. Based on the Bradford Hill criteria, delta-9 tetrahydrocannabinol (THC) may be the causative xenobiotic for this phenomenon.

Similar content being viewed by others
Change history
21 April 2022
A Correction to this paper has been published: https://doi.org/10.1007/s13181-022-00895-2
02 November 2022
A Correction to this paper has been published: https://doi.org/10.1007/s13181-022-00915-1
References
Bridgeman MB, Abazia DT. Medicinal cannabis: history, pharmacology, and implications for the acute care setting. P T. 2017;42(3):180–8.
Barrus DG Capogrossi KL Cates SC Gourdet CK Peiper NC Novak SP et al. Tasty THC: promises and challenges of cannabis edibles. Methods Rep RTI Press. 2016;2016.
Monte AA, Shelton SK, Mills E, Saben J, Hopkinson A, Sonn B, et al. Acute illness associated with cannabis use, by route of exposure. Ann Intern Med. 2019;170(8):531–7.
Vandrey R, Raber JC, Raber ME, Douglass B, Miller C, Bonn-Miller MO. Cannabinoid dose and label accuracy in edible medical cannabis products. JAMA. 2015;313(24):2491–3.
Hammond D. Communicating THC levels and ‘dose’ to consumers: implications for product labelling and packaging of cannabis products in regulated markets. International J of Drug Policy. 2021;91.
Freeman TP, Lorenzetti V. ‘Standard THC units’: a proposal to standardize dose across all cannabis products and methods of administration. Addiction. 2020;115(7):1207–16.
Gounder K Dunuwille J Dunne J Lee J Silbert P Lawn N. The other side of the leaf: seizures associated with synthetic cannabinoid use. Epilepsy Behav. 2020;104(Pt A):106901.
Kemp AM Clark MS Dobbs T Galli R Sherman J Cox R. Top 10 facts you need to know about synthetic cannabinoids: not so nice spice. Am J Med. 2016;129(3):240–4 e1.
Lapoint JM. Cannabinoids. In: Nelson LS, Howland MA, Lewin NA, Smith SW, Goldfrank LR, Hoffman RS, editors. Goldfrank’s toxicologic emergencies, 11e. New York, NY: McGraw-Hill Education; 2019. p. 1111–23.
Spindle TR, Bonn-Miller MO, Vandrey R. Changing landscape of cannabis: novel products, formulations, and methods of administration. Curr Opin Psychology. 2019;30:98–102.
Espejo-Porras F, Fernández-Ruiz J, Pertwee RG, Mechoulam R, García C. Motor effects of the non-psychotropic phytocannabinoid cannabidiol that are mediated by 5-HT1A receptors. Neuropharmacol. 2013;75:155–63.
Morales P, Reggio PH. An Update on Non-CB1, Non-CB2 cannabinoid related G-protein-coupled receptors. Cannabis and Cannabinoid Research. 2017;2(1):265–73.
Navarro G, Varani K, Lillo A, Vincenzi F, Rivas-Santisteban R, Raïch I, et al. Pharmacological data of cannabidiol- and cannabigerol-type phytocannabinoids acting on cannabinoid CB1, CB2 and CB1/CB2 heteromer receptors. Pharmacol Res. 2020;159:104940.
Rosenthaler S, Pöhn B, Kolmanz C, Nguyen Huu C, Krewenka C, Huber A, et al. Differences in receptor binding affinity of several phytocannabinoids do not explain their effects on neural cell cultures. Neurotoxicol Teratol. 2014;46:49–56.
Le Boisselier R, Alexandre J, Lelong-Boulouard V, Debruyne D. Focus on cannabinoids and synthetic cannabinoids. Clin Pharmacol Ther. 2017;101(2):220–9.
Abuhasira R, Shbiro L, Landschaft Y. Medical use of cannabis and cannabinoids containing products - regulations in Europe and North America. Eur J Intern Med. 2018;49:2–6.
Zoorob MJ. The frequency distribution of reported THC concentrations of legal cannabis flower products increases discontinuously around the 20% THC threshold in Nevada and Washington state. J of Cannabis Res. 2021;3(1):6.
Barrus D Capogrossi K. Cates S. Gourdet C. Peiper N. Novak S. Lefever T. & Wiley J. Tasty THC: promises and challenges of cannabis edibles. RTI Press Occasional Paper 2016.
Morrison PD, Murray RM. Cannabis points to the synaptic pathology of mental disorders: how aberrant synaptic components disrupt the highest psychological functions. Dialogues Clin Neurosci. 2020;22(3):251–8.
Bonn-Miller MO, Loflin MJE, Thomas BF, Marcu JP, Hyke T, Vandrey R. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318(17):1708–9.
Russo EB McPartland JM. Cannabis is more than simply delta(9)-tetrahydrocannabinol. Psychopharmacology (Berl). 2003;165(4):431–2; author reply 3–4.
Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol. 2011;163(7):1344–64.
Burrows K, Williams JA. THC intoxication in a 16-month-old child. Paediatr Child Health. 2019;24(5):299–300.
Silliman Cohen R, Barron CE, Goldberg A. Altered mental status in a young child: a case of child neglect. Clin Pediatr (Phila). 2019;58(1):123–5.
Carvalho A, Evans-Gilbert T. The pathophysiology of marijuana-induced encephalopathy and possible epilepsy after ingestion in children: a case series. Innov Clin Neurosci. 2019;16(3–4):16–8.
Emoto J, Weeks K, Kallail KJ. Accidental acute cannabis intoxication presenting as seizure in pediatrics patients. Kans J Med. 2020;13:129–30.
Boadu O, Gombolay GY, Caviness VS, El Saleeby CM. Intoxication from accidental marijuana ingestion in pediatric patients: what may lie ahead. Pediatr Emerg Care. 2020;36(6):e349–54.
Bonkowsky JL, Sarco D, Pomeroy SL. Ataxia and shaking in a 2-year-old girl: acute marijuana intoxication presenting as seizure. Pediatr Emerg Care. 2005;21(8):527–8.
Richards JR, Smith NE, Moulin AK. Unintentional cannabis ingestion in children: a systematic review. J Pediatr. 2017;190:142–52.
Singh D, Huntwork M, Shetty V, Sequeira G, Akingbola O. Prolonged atrial fibrillation precipitated by new-onset seizures and marijuana abuse. Pediatrics. 2014;133(2):e443–6.
Fogang YF, Camara M, Mbonda PC, Toffa D, Toure K. Late onset epilepsy associated with marijuana abuse: a case report with MRI findings. Pan Afr Med J. 2014;17:158.
Brust JC, Ng SK, Hauser AW, Susser M. Marijuana use and the risk of new onset seizures. Trans Am Clin Climatol Assoc. 1992;103:176–81.
Stockings E, Zagic D, Campbell G, Weier M, Hall WD, Nielsen S, et al. Evidence for cannabis and cannabinoids for epilepsy: a systematic review of controlled and observational evidence. J Neurol Neurosurg Psychiatry. 2018;89(7):741–53.
Meiri E, Jhangiani H, Vredenburgh JJ, Barbato LM, Carter FJ, Yang H-M, et al. Efficacy of dronabinol alone and in combination with ondansetron versus ondansetron alone for delayed chemotherapy-induced nausea and vomiting. Curr Med Res Opin. 2007;23(3):533–43.
May MB, Glode AE. Dronabinol for chemotherapy-induced nausea and vomiting unresponsive to antiemetics. Cancer Manag Res. 2016;8:49–55.
Beal JE, Olson R, Laubenstein L, Morales JO, Bellman P, Yangco B, et al. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage. 1995;10(2):89–97.
Beal JE, Olson R, Lefkowitz L, Laubenstein L, Bellman P, Yangco B, et al. Long-term efficacy and safety of dronabinol for acquired immunodeficiency syndrome-associated anorexia. J Pain Symptom Manage. 1997;14(1):7–14.
Rintala DH, Fiess RN, Tan G, Holmes SA, Bruel BM. Effect of dronabinol on central neuropathic pain after spinal cord injury: a pilot study. Am J Phys Med Rehabil. 2010;89(10):840–8.
Lofwall MR, Babalonis S, Nuzzo PA, Elayi SC, Walsh SL. Opioid withdrawal suppression efficacy of oral dronabinol in opioid dependent humans. Drug Alcohol Depend. 2016;164:143–50.
Narang S, Gibson D, Wasan AD, Ross EL, Michna E, Nedeljkovic SS, et al. Efficacy of dronabinol as an adjuvant treatment for chronic pain patients on opioid therapy. J of Pain. 2008;9(3):254–64.
Lorenz R. On the application of cannabis in paediatrics and epileptology. Neuro Endocrinol Lett. 2004;25:40–4.
Woodward MR, Harper DG, Stolyar A, Forester BP, Ellison JM. Dronabinol for the treatment of agitation and aggressive behavior in acutely hospitalized severely demented patients with noncognitive behavioral symptoms. Am J Geriatr Psychiatry. 2014;22(4):415–9.
Kuhlen M, Hoell JI, Gagnon G, Balzer S, Oommen PT, Borkhardt A, et al. Effective treatment of spasticity using dronabinol in pediatric palliative care. European J of Paediatric Neurology. 2016;20(6):898–903.
Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodology. 2018;18(1):143.
Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J. 2009;26(2):91–108.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73.
Guidet C, Gregoire M, Le Dreau A, Vrignaud B, Deslandes G, Monteil-Ganiere C. Cannabis intoxication after accidental ingestion in infants: urine and plasma concentrations of Delta-9-tetrahydrocannabinol (THC), THC-COOH and 11-OH-THC in 10 patients. Clin Toxicol (Phila). 2020;58(5):421–3.
Desai R, Shamim S, Patel K, Sadolikar A, Kaur VP, Bhivandkar S, et al. Primary causes of hospitalizations and procedures, predictors of in-hospital mortality, and trends in cardiovascular and cerebrovascular events among recreational marijuana users: a five-year nationwide inpatient assessment in the United States. Cureus. 2018;10(8):e3195.
Heizer JW, Borgelt LM, Bashqoy F, Wang GS, Reiter PD. Marijuana misadventures in children: exploration of a dose-response relationship and summary of clinical effects and outcomes. Pediatr Emerg Care. 2018;34(7):457–62.
Wolfe CE, Wood DM, Dines A, Whatley BP, Yates C, Heyerdahl F, et al. Seizures as a complication of recreational drug use: analysis of the Euro-DEN Plus data-set. Neurotoxicology. 2019;73:183–7.
Schmid Y, Scholz I, Mueller L, Exadaktylos AK, Ceschi A, Liechti ME, et al. Emergency department presentations related to acute toxicity following recreational use of cannabis products in Switzerland. Drug Alcohol Depend. 2020;206:107726.
Noble MJ, Hedberg K, Hendrickson RG. Acute cannabis toxicity. Clin Toxicol (Phila). 2019;57(8):735–42.
Wang GS, Roosevelt G, Heard K. Pediatric marijuana exposures in a medical marijuana state. JAMA Pediatr. 2013;167(7):630–3.
Claudet I, Le Breton M, Brehin C, Franchitto N. A 10-year review of cannabis exposure in children under 3-years of age: do we need a more global approach? Eur J Pediatr. 2017;176(4):553–6.
Liakoni E Dolder PC Rentsch K Liechti ME Acute health problems due to recreational drug use in patients presenting to an urban emergency department in Switzerland. Swiss Med Wkly. 2015;145:w14166.
Onders B, Casavant MJ, Spiller HA, Chounthirath T, Smith GA. Marijuana exposure among children younger than six years in the United States. Clin Pediatr (Phila). 2016;55(5):428–36.
Anderson SAR Oprescu AM Calello DP Monte A Dayan PS Hurd YL et al. Neuropsychiatric sequelae in adolescents with acute synthetic cannabinoid toxicity. Pediatrics. 2019;144(2).
Graham J Leonard J Banerji S Wang GS Illicit drug exposures in young pediatric patients reported to the National Poison Data System, 2006–2016. J Pediatr. 2020;219:254–8 e1.
Onders B, Casavant MJ, Spiller HA, Chounthirath T, Smith GA. Marijuana exposure among children younger than six years in the United States. Clin Pediatr. 2015;55(5):428–36.
Patel RS, Mekala HM, Tankersley WE. Cannabis use disorder and epilepsy: a cross-national analysis of 657 072 hospitalized patients. Am J Addict. 2019;28(5):353–60.
Desai R, Patel U, Sharma S, Amin P, Bhuva R, Patel MS, et al. Recreational marijuana use and acute myocardial infarction: insights from nationwide inpatient sample in the United States. Cureus. 2017;9(11):e1816.
Huff JS, Morris DL, Kothari RU, Gibbs MA, Emergency Medicine Seizure Study G. Emergency department management of patients with seizures: a multicenter study. Acad Emerg Med. 2001;8(6):622–8.
Krumholz A, Grufferman S, Orr ST, Stern BJ. Seizures and seizure care in an emergency department. Epilepsia. 1989;30(2):175–81.
Pallin DJ, Goldstein JN, Moussally JS, Pelletier AJ, Green AR, Camargo CA Jr. Seizure visits in US emergency departments: epidemiology and potential disparities in care. Int J Emerg Med. 2008;1(2):97–105.
Schoenenberger RA, Heim SM. Indication for computed tomography of the brain in patients with first uncomplicated generalised seizure. BMJ. 1994;309(6960):986–9.
Zack MM, Kobau R. National and state estimates of the numbers of adults and children with active epilepsy - United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(31):821–5.
Fiest KM, Sauro KM, Wiebe S, Patten SB, Kwon CS, Dykeman J, et al. Prevalence and incidence of epilepsy: a systematic review and meta-analysis of international studies. Neurology. 2017;88(3):296–303.
Camfield P, Camfield C. Incidence, prevalence and aetiology of seizures and epilepsy in children. Epileptic Disord. 2015;17(2):117–23.
ElSohly MA, Ross SA, Mehmedic Z, Arafat R, Yi B, Banahan BF 3rd. Potency trends of delta9-THC and other cannabinoids in confiscated marijuana from 1980–1997. J Forensic Sci. 2000;45(1):24–30.
Stambouli H, El Bouri A, Bouayoun T. Évolution de la teneur en Δ9-THC dans les saisies de résines de cannabis au Maroc de 2005 à 2014. Toxicol Anal et Clin. 2016;28(2):146–52.
Ham MT, Loskota WJ, Lomax P. Acute and chronic effects of Δ9-tetrahydrocannabinol on seizures in the gerbil. Euro J Pharmacol. 1975;31(1):148–52.
Consroe P, Martin AR, Fish BS. Use of a potential rabbit model for structure–behavioral activity studies of cannabinoids. J Med Chem. 1982;25(5):596–9.
Johnson DD McNeill JR Crawford RD Wilcox WC Epileptiform seizures in domestic fowl. V. The anticonvulsant activity of Δ9-tetrahydrocannabinol. Canadian Journal of Physiology and Pharmacology. 1975;53(6):1007–13.
Chiu P, Olsen DM, Borys HK, Karler R, Turkanis SA. The influence of cannabidiol and delta 9-tetrahydrocannabinol on cobalt epilepsy in rats. Epilepsia. 1979;20(4):365–75.
Colasanti BK, Lindamood C 3rd, Craig CR. Effects of marihuana cannabinoids on seizure activity in cobalt-epileptic rats. Pharmacol Biochem Behav. 1982;16(4):573–8.
Turkanis SA, Karler R. Central excitatory properties of Δ9-tetrahydrocannabinol and its metabolites in iron-induced epileptic rats. Neuropharmacol. 1982;21(1):7–13.
Karler R, Calder L, Sangdee P, Turkanis S. Interaction between delta-9-tetrahydro-cannabinol and kindling by electrical and chemical stimuli in mice. Neuropharmacol. 1984;23(11):1315–20.
Turkanis SA, Karler R. Electrophysiologic properties of the cannabinoids. J Clin Pharmacol. 1981;21(S1):449S-S463.
Wada JA, Wake A, Sato M, Corcoran ME. Antiepileptic and prophylactic effects of tetrahydrocannabinols in amygdaloid kindled cats. Epilepsia. 1975;16(3):503–10.
Sofia RD, Solomon TA, Barry H 3rd. Anticonvulsant activity of delta9-tetrahydrocannabinol compared with three other drugs. Eur J Pharmacol. 1976;35(1):7–16.
Culler CA, Vigani A. Successful treatment of a severe cannabinoid toxicity using extracorporeal therapy in a dog. J Vet Emerg Crit Care (San Antonio). 2019;29(6):674–9.
Girling SJ, Fraser MA. Cannabis intoxication in three Green iguanas (Iguana iguana). J Small Anim Pract. 2011;52(2):113–6.
Brutlag A, Hommerding H. Toxicology of marijuana, synthetic cannabinoids, and cannabidiol in dogs and cats. Vet Clin North Am Small Anim Pract. 2018;48(6):1087–102.
Breivogel CS, Wells JR, Jonas A, Mistry AH, Gravley ML, Patel RM, et al. Comparison of the neurotoxic and seizure-inducing effects of synthetic and endogenous cannabinoids with delta(9)-tetrahydrocannabinol. Cannabis Cannabinoid Res. 2020;5(1):32–41.
Malyshevskaya O, Aritake K, Kaushik MK, Uchiyama N, Cherasse Y, Kikura-Hanajiri R, et al. Natural ((9)-THC) and synthetic (JWH-018) cannabinoids induce seizures by acting through the cannabinoid CB1 receptor. Sci Rep. 2017;7(1):10516.
Whalley BJ, Lin H, Bell L, Hill T, Patel A, Gray RA, et al. Species-specific susceptibility to cannabis-induced convulsions. Br J Pharmacol. 2019;176(10):1506–23.
Kaczor EE, Mathews B, LaBarge K, Chapman BP, Carreiro S. Cannabis product ingestions in pediatric patients: ranges of exposure, effects, and outcomes. J Med Toxicol. 2021;17(4):386–96.
Fedak KM, Bernal A, Capshaw ZA, Gross S. Applying the Bradford Hill criteria in the 21st century: how data integration has changed causal inference in molecular epidemiology. Emerg Themes Epidemiol. 2015;12(1):14.
Kreitzer AC. Neurotransmission: emerging roles of endocannabinoids. Curr Biol. 2005;15(14):R549–51.
Katona I, Freund TF. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med. 2008;14(9):923–30.
Wiley JL, Lefever TW, Marusich JA, Grabenauer M, Moore KN, Huffman JW, et al. Evaluation of first generation synthetic cannabinoids on binding at non-cannabinoid receptors and in a battery of in vivo assays in mice. Neuropharmacol. 2016;110:143–53.
Schneir AB, Baumbacher T. Convulsions associated with the use of a synthetic cannabinoid product. J Med Toxicol. 2012;8(1):62–4.
Funada M, Takebayashi-Ohsawa M. Synthetic cannabinoid AM2201 induces seizures: involvement of cannabinoid CB1 receptors and glutamatergic transmission. Toxicol and Appl Pharmacol. 2018;338:1–8.
Chen HY, Albertson TE, Olson KR. Treatment of drug-induced seizures. Br J Clin Pharmacol. 2016;81(3):412–9.
Holtkamp M. Pharmacotherapy for refractory and super-refractory status epilepticus in adults. Drugs. 2018;78(3):307–26.
Thomas AA, Dickerson-Young T, Mazor S. Unintentional pediatric marijuana exposures at a tertiary care children’s hospital in Washington State: a retrospective review. Pediatr Emerg Care. 2021;37(10):e594–8.
Thomas AA, Mazor S. Unintentional marijuana exposure presenting as altered mental status in the pediatric emergency department: a case series. J Emerg Med. 2017;53(6):e119–23.
Toce MS, Farias M, Powell AJ, Daly KP, Vargas SO, Burns MM. Myocardial infarct after marijuana inhalation in a 16-year-old adolescent boy. Pediatr Dev Pathol. 2019;22(1):80–6.
Wadhwa A, Bal S, Imoukhuede O, Doshi D. Shattering the carotids. Stroke. 2021;52(5):e160–3.
Acknowledgements
The authors would like to thank Catherine Carr, medical research librarian, for her assistance with the literature search.
Funding
This work was partially funded by NIH/NIDA K23DA045242 (PI: Carreiro).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. EK, Dr. KG, Dr. JZ, and Dr. MN declare that they have no conflict of interest.
Dr. LT has received honoraria for speaking and consultation fees for expert testimony both unrelated to this work.
Dr. SC is funded by the NIH/NIDA (K23DA045242).
Additional information
Supervising Editor: Michael Hodgman, MD.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: The surname of coauthor Stephanie Carreiro was spelled incorrectly (as “Carriero”) in this article as originally published.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaczor, E.E., Greene, K., Zacharia, J. et al. The Potential Proconvulsant Effects of Cannabis: a Scoping Review. J. Med. Toxicol. 18, 223–234 (2022). https://doi.org/10.1007/s13181-022-00886-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13181-022-00886-3