Abstract
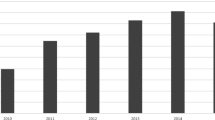
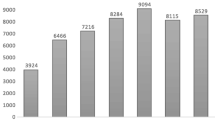
The Toxicology Investigators Consortium (ToxIC) Case Registry was established in 2010 by the American College of Medical Toxicology. The Registry includes all medical toxicology consultations performed at participating sites. This report summarizes the Registry data for 2013. A query of the ToxIC Registry was carried out for the dates of January 1 through December 31, 2013. Specific data reviewed for analysis included demographics (age, gender), source of consultation, reasons for consultation, agents involved in toxicological exposures, signs, symptoms and clinical findings, and treatment. A total of 8,598 cases were entered into the Registry in 2013. Females accounted for 49.2 % of cases, males for 47.7 %, and gender was not reported in 3.1 %. The majority of patients (63.4 %) were adults between the ages of 19 and 65 years. There were 93 fatalities (1.1 %). Most referrals for medical toxicology consultation originated from the emergency department (59.7 %) or inpatient services (16.7 %). Exposures to pharmaceutical products (intentional and unintentional) made up 50.0 % of cases. Illicit drug abuse (8.0 %) and adverse drug reactions (ADRs) (4.8 %) were the next most frequent reasons for consultation. Similar to past years, nonopioid analgesics, sedative-hypnotics, and opioids were the most commonly encountered agents. Symptoms or clinical findings were documented in 71.1 % of patients. Of all cases, 54.6 % required some form of medical treatment (antidotes, antivenom, chelation, specific types of supportive care). This report serves as a comprehensive survey of medical toxicology practice within participating institutions. Prior trends continued to apply this year and indicate analgesic (opioid and nonopioid), sedative-hypnotic/muscle relaxant agents, illicit drug use, and ADRs continue to be major toxicological problems. Cases requiring medical toxicology consultation in 2013 predominantly involved pharmaceuticals and illicit drugs. Reasons for these drug exposures were diverse and included intentional overdose, unintentional exposure, withdrawal syndromes, and ADRs. Nonopioid analgesics, sedative-hypnotic agents, and opioids remained the most frequently encountered agent classes. While over half of cases required some form of medical treatment, fatalities were uncommon.

Similar content being viewed by others
References
Wax PM, Kleinschmidt KC, Brent J (2011) The Toxicology Investigators Consortium (ToxIC) Registry. J Med Toxicol 7(4):259–265
Brent J, Wax PM, Schwartz T et al (2011) The Toxicology Investigators Consortium Case Registry—the 2010 experience. J Med Toxicol 7(4):266–276
Wiegand T, Wax P, Smith E et al (2013) The Toxicology Investigators Consortium Case Registry—the 2012 experience. J Med Toxicol 9(4):380–404
Wiegand TJ, Wax PM, Schwartz T et al (2012) The Toxicology Investigators Consortium Case Registry—the 2011 experience. J Med Toxicol 8(4):360–377
Acknowledgments
The authors express their sincere gratitude to the staff at the American College of Medical Toxicology for supporting the ToxIC Registry project. We very much appreciate the contributions to the Registry from each of the ToxIC sites. The following is a list of the principle coordinators from each site:
Boston, MA (Beth Israel Deaconess Medical Center)
Michael Ganetsky, MD
Boston, MA (Children’s Hospital of Boston)
Michele Burns-Ewald, MD
Charlotte, NC
Michael Beuhler, MD
Charlottesville, VA
Joshua King, MD
Chicago, IL
Steven Aks, DO
Cincinnati, OH
Shan Yin, MD
Dallas, TX
Kurt Kleinschmidt, MD
Paul Wax, MD
Denver, CO
Jeffrey Brent, MD
Eric Lavonas, MD
Evanston, IL
Jerold Leikin, MD
Fresno, CA
Rais Vohra, MD
Grand Rapids, MI
Bryan Judge, MD
Bradley Riley, MD
Haifa, Israel
Yedidia Bentur, MD
Harrisburg, PA
J. Ward Donovan, MD
Hartford, CT
Charles McKay, MD
Houston, TX
Spencer Greene, D
Indianapolis, IN
Daniel Rusyniak, MD
Kansas City, MO
Jennifer Lowry, MD
D. Adam Algren, MD
Los Angeles, CA
Michael Levine, MD
Manhasset, NY
Josh Nogar, MD
Milwaukee, WI
David Gummin, MD
Mark Kostic, MD
Morristown, NJ
Diane Calello, MD
New Brunswick, NJ
Ann-Jeanette Geib, MD
Newark, NJ
Steven Marcus, MD
New York, NY (Mt. Sinai Hospital)
Stephanie Hernandez, MD
Alex Manini, MD
New York, NY (NYU Langone Medical Center)
Silas Smith, MD
Lewis Nelson, MD
New York, NY (Staten Island University Hospital)
Nima Majlesi, DO
Omaha, NE
Ronald Kirschner, MD
Philadelphia (Hahnemann University Hospital)
David Vearrier, MD
Philadelphia (Einstein Medical Center)
Adam K. Rowden, DO
Phoenix, AZ
Michael Levine, MD
Anne-Michelle Ruha, MD
Pittsburgh, PA
Anthony Pizon, MD
Portland, OR
Nathaneal McKeown, DO
Richmond, VA
Brandon Wills, DO
Kirk Cumpston, DO
Rochester, NY
Timothy Wiegand, MD
Salt Lake City, UT
E. Martin Caravati, MD
San Antonio, TX
Shawn Varney, MD
Vikhyat Bebarta, MD
San Francisco, CA
Derrick Lung, MD
Craig Smollin, MD
St. Louis, MO
Evan Schwarz, MD
Thomas Kibby, MD
St. Paul, MN
Samuel Stellpflug, MD
Kristin Engebretsen, PharmD
Sydney West, Australia
Naren Gunga, MD
Worcester, MA
Sean Rhyee, MD, MPH
Conflict of Interest
The authors have no conflicts of interests to disclose relevant to the content of this manuscript. No outside funding was involved in the production of this manuscript.
Funding Sources
Support for the Registry in 2013 was derived from three sources: NINDS grant U01 NS083452-01S1, an unrestricted grant from BTG International Ltd., and the American College of Medical Toxicology, the sponsor of ToxIC.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Data contained in this manuscript has not been previously presented in any form.
Rights and permissions
About this article
Cite this article
Rhyee, S.H., Farrugia, L., Wiegand, T. et al. The Toxicology Investigators Consortium Case Registry—the 2013 Experience. J. Med. Toxicol. 10, 342–359 (2014). https://doi.org/10.1007/s13181-014-0417-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13181-014-0417-0