Abstract
Some groups of invertebrates from intermittent wetlands produce dormant stages in response to environmental fluctuations. Dormancy is a strategy to survive such fluctuations and to persist in extreme aquatic habitats, such as temporary habitats. We investigated the hatching responses of invertebrate dormant stages across different depths of sediment in intermittent ponds. Our hypotheses were: (1) the richness and abundance of invertebrate hatchlings decrease as the depth of the sediment column increases, and (2) the composition of invertebrate hatchlings varies over the wetland sediment depth. Four intermittent ponds were sampled in southern Brazil. One sediment column of 30 cm depth was collected in each pond and stratified into 1 cm thick slices for analysis of the dormant stages. A total of 1,931 hatchlings distributed among 31 taxa were collected from the sediment columns over the experiment. The total richness and abundance of hatchlings (after bdelloid taxa exclusion) were negatively related with the sediment depth. The composition of aquatic invertebrates varied among the different strata over the sediment depth. As intermittent wetlands are ecosystems extremely susceptible to climate variations, the results help to understand the resilience of aquatic resistant communities from different sediment strata after drought events.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Some groups of invertebrates from intermittent wetlands produce dormant stages in response to environmental fluctuations (Brendonck and De Meester 2003; Parra et al. 2021), such as resistant eggs and cysts (Williams 2006). Dormancy is a strategy to survive such fluctuations and to persist in extreme aquatic habitats (Santangelo 2009; Strachan et al. 2015; García-Roger et al. 2017). Several taxa of invertebrate use dormancy as a survival strategy, and wetland sediments may contain thousands of dormant stages of different species per square meter, particularly rotifers, cladocerans and copepods (Hairston 1996; Panarelli et al. 2008; Day et al. 2010; Brendonck et al. 2017).
The production and hatching of invertebrate dormant stages in intermittent wetlands are influenced by several environmental variables. Frequency and length of hydroperiod are important variables to hatching patterns and production of invertebrate dormant stages (Nielsen et al. 2000; Vargas et al. 2019; da Silva Bandeira et al. 2020). Temperature and photoperiod are also key factors for invertebrate hatching patterns (Gyllström and Hansson 2004; Wang and Chou 2015). Water chemistry factors (e.g., salinity, conductivity and dissolved oxygen) are also important abiotic hatching cues (Brendonck 1996; Vanschoenwinkel et al. 2010).
The bank of dormant stages of aquatic invertebrates is a historical ecological archive, formed by the overlap of several generations, which allows correlating the dynamics of communities to environmental changes (Brendonck and De Meester 2003; Rogalski 2015; Rogalski et al. 2017; García-Roger and Ortells 2018). For instance, the hatching of dormant stages of rotifers, cladocerans and copepods after decades of dormancy has been reported in sediments from the estuary of the Pettaquamscutt River in Rhode Island, USA (Marcus et al. 1994). There are examples of even older hatchings, such as copepods around 300 years old (Hairston et al. 1995), cladoceran genetic material dated to 600 years old (Frisch et al. 2014) and a bdelloid rotifer of 24,000 years dormant in the permafrost of the Alazeya River in Siberia, Russia (Shmakova et al. 2021). This feature can be used as an important ecological and evolutionary tool for studies of dormant aquatic invertebrate communities (Brendonck and De Meester 2003; Angeler 2007).
Studies indicate that the upper layer of the sediment (between 4 and 10 cm) has the highest concentrations of viable dormant stages (Herzig 1985; Cáceres 1998; Cáceres and Hairston 1998; Hairston et al. 2000; Santangelo 2009). Therefore, the unhatched dormant stages accumulate at greater depths over time (Ellner and Hairston 1994; Brendonck and De Meester 2003), and the hatching rate tends to decrease with depth (Hairston et al. 1999; Kerfoot et al.1999). Nonetheless, studies correlating the viability of dormant stages and the depth of the sediment were carried out mainly in intermittent wetlands in temperate regions of Europe and North America (Herzig 1985; Hairston et al. 1995; Kerfoot et al.1999; Gyllström and Hansson 2004). This relationship was poorly studied in Neotropical region (Iglesias et al. 2016). The studies that report the presence of dormant stages in southern and southeastern Brazil wetlands (Maia-Barbosa et al. 2003; Stenert et al. 2010, 2016, 2017; Santangelo et al. 2014; Ávila et al. 2015; Freiry et al. 2016, 2020a, b; Vargas et al. 2019; Brazil et al. 2022) only analyzed the top layers of the sediment (3–5 cm).
Here, we investigated the hatching responses of invertebrate dormant stages across different depths of sediment in intermittent ponds. The objectives were to: (1) evaluate the richness, abundance and composition of hatched invertebrates along a vertical gradient of the sediment column, and (2) compare the hatching of the main taxonomic groups along the sediment column. Our first hypothesis is that the richness and abundance of invertebrate hatchlings decrease as the depth of the sediment column increases since the largest fraction of viable and more responsive dormant stages occurs in the top layers of the sediment (Hairston et al. 2000; Brendonck and De Meester 2003; Yousey et al. 2018). Our second hypothesis is that the composition of invertebrate hatchlings varies over the wetland sediment depth considering that several invertebrate taxa use dormancy as a survival strategy (Brendonck et al. 2017) and that not all dormant stages have the same ability to survive for long in the egg bank (Hairston 1996).
Materials and methods
Study Area
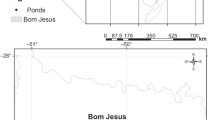
This study was conducted in the Coastal Plain of Southern Brazil, a region extending across approximately 640 km with a high concentration of wetlands (Maltchik et al. 2003) (Fig. 1). The climate is moist subtropical with a mean annual temperature of 17.5 ◦C, and the annual mean rainfall ranges from 1,200 to 1,500 mm (Rambo 2000). The flat topography of the landscape and the low altitudes (lower than 20 m) makes climate conditions very similar throughout the study region.
Sampling Design
Four intermittent depressional ponds were sampled in April 2019 (Fig. 1). The isolated ponds analyzed were intermittent, with similar sizes (1 ha) and water depth (0.5 m on average) distant at least 15 km from each other. On each pond, one sediment column of 30 cm depth was collected using a Russian peat borer (Modelo 2460-F20) (5-cm diameter). To collect the sediment, the tube corer was pushed vertically, avoiding disturbance in the deeper part of the sediments. The sediment sampling was carried out when the ponds had water. Each sediment column was transferred to a PVC corer adapted to receive the sediment column, without alteration. The samples were kept in darkness by wrapping in aluminum foil and refrigerated (4 ◦C) until experiments start (Cousyn and De Meester 1998; García-Roger and Ortells 2018). Data collection complied with the current Brazilian environmental laws (SISBIO 36365-2).
Laboratory Procedures
Each sediment column was stratified into 1 cm thick slices for analysis of the dormant stages. Since the upper centimeters of the sediment contains the active egg bank, the slice thickness varied with depth, with smallest intervals (1 cm) in the top layers (up to 6 cm) and 4 cm for deeper sediment layers (Hairston et al. 1995, 2000; Kerfoot et al. 1999; Brendonck and De Meester 2003). In total, eleven slices per sediment column (pond) were incubated in our experiment all in the same period (0-1 cm, 1–2 cm, 2–3 cm, 3–4 cm, 4–5 cm, 5–6 cm, 10–11 cm, 15–16 cm, 20–21 cm, 25–26 cm e 30–31 cm), totalizing 44 samples (11 samples x 4 selected ponds). All slices were dehydrated in a dark oven for 96 h at 40 °C.
In the incubation experiment, each sediment slice was submersed under a depth of 2 cm of distilled water into plastic trays without aerators, and water temperature (23 ± 2 ºC) and photoperiod (12 h light/12 hours dark) were kept constant (Ávila et al. 2015; Stenert et al. 2010). The experiment was maintained in the laboratory for 4 weeks (June 7th to July 5th, 2019), and hatchlings were collected three times per week, corresponding to 12 sampling days, by filtering all the water content from each plastic tray through a 50-µm mesh size net. The collected hatchlings were transferred to 1.5-mL polypropylene microtubes with 80% alcohol or 4% formaldehyde (Rotifera) (Freiry et al. 2016). The distilled water was changed after each sampling day. The duration of the experiment (4 weeks) was based on previous work from our research group (Freiry et al. 2020b; Vendramin et al. 2020) and others (Brock et al. 2003). Hatchlings were quantified under stereomicroscope (Zeiss Stemi 2000) and identified to species level whenever possible using specialized literature (Koste 1978; Elmoor-Loureiro 1997; Gazulha 2012) and aid of specialists.
Data Analyses
The richness and abundance of aquatic invertebrates were the taxa number (number of species or genus – whenever possible – added to the number of taxa identified at lower taxonomic resolutions – phylum, class, or family level) and number of hatchlings, respectively. The relationship of invertebrate richness and abundance with different sediment depths was tested with generalized linear models (GLMs). As both response variables were discrete, GLMs were fitted with Poisson (richness) and negative binomial (abundance) distributions (because of major overdispersion of residuals) and log link function. The predictor variable (sediment depth) was included in the models as a numerical variable. The models were tested for richness and abundance of the total community.
Each sediment column was divided into three different strata: superficial (from 1 to 5 cm, represented by five slices – 1, 2, 3, 4 and 5 cm), intermediate (from 6 to 20 cm, represented by three slices – 6, 11 and 16 cm) and deep (from 21 to 31 cm, represented by three slices – 21, 26 and 31 cm) to analyze the spatial variation in the composition of hatchlings. The spatial variation in the composition of aquatic invertebrates among the different depth strata was assessed using nonmetric multidimensional scaling ordination diagram (NMDS) and a nonparametric multivariate analysis of variance (PERMANOVA) with 9,999 permutations. The NMDS and PERMANOVA analyses were based on an incidence matrix (Jaccard index). A similarity percentage analysis (SIMPER; Clarke 1993; 999 permutations) was used to identify the taxa that mostly contributed to differences among depth strata. We used the PERMDISP approach (betadisper function) (Anderson 2006) to test for differences in the multivariate dispersion among the sediment depth strata. All statistical analyses were conducted with the functions from packages vegan, car, MASS, lme4 and ggplot2 in the R software v. 4.0.3 (R Development Core Team 2020). The Panplot2 portable software (Sieger and Grobe 2013) and CorelDRAW were used to visualize the total hatching percentage of the main invertebrate taxa in relation to sediment depth.
Results
A total of 1,931 hatchlings distributed among 31 taxa were collected from the sediment columns over the experiment. Phylum Rotifera comprised most of the hatchlings (82%), followed by the Phylum Gastrotricha (8%) and Phylum Annelida (Family Aeolosomatidae − 6%). The Subphylum Crustacea (2.5%) was represented by the Class Ostracoda (1.8%), Subclass Copepoda (only 1 nauplius – 0.05%) and Order Anomopoda (Cladocera, 13 individuals – 0.7%). Phylum Nematoda (1.2%), Phylum Tardigrada (0.4%) and Phylum Platyhelminthes (class Turbellaria – microturbellarians – 0.3%) corresponded to the rest of the hatched individuals. The most abundant taxa were the bdelloid rotifers Philodina sp. (52%) and Adineta sp. (12%), and the monogonont rotifers Lecane leontina Turner, 1892 (9%) and Ptygura pilula (7%) (Table 1). Although the highest percentage of hatchings of these taxa occurred in the surface layers of sediment, some individuals hatched at depths greater than 20 cm (Table 1; Fig. 2).
The total richness of taxa was negatively influenced by the sediment depth, since the hatchings showed greater richness in the top layers compared to the deeper ones (Z = -5.533; p < 0.001; Fig. 3). The total hatching abundance was not influenced by depth (Z = -1.197; p = 0.231) (Fig. 4). Considering that the dominance of the Bdelloidea rotifers could influence variation in abundance, we excluded these taxa and reanalyzed the data. After bdelloid taxa exclusion, the abundance was also negatively influenced by the sediment depth (Z = -5.460; p < 0.001) (Fig. 5).
The composition of aquatic invertebrates varied among the different strata over the sediment depth (PERMANOVA, F2,41 = 3.022; p < 0.001) and this variation was displayed by two axes of the NMDS diagram (stress = 0.125) (Fig. 6). The PERMANOVA results were not affected by multivariate dispersion within the sediment depth strata (F2,41 = 0.846; p = 0.446). The similarity percentage (SIMPER) analysis revealed that nine taxa significantly contributed to the dissimilarity in the composition of the different strata, and the taxa with the highest contribution were Adineta sp., Ptygura pilula, Ostracoda, Gastrotricha and Aeolosomatidae (Online Resource 1).
Nonmetric multidimensional scaling ordination for aquatic invertebrate hatchlings from superficial, intermediate and deep strata along the sediment of the studied ponds. The red crosses (+) are the invertebrate taxa; the filled black dots (●) corresponded to the superficial stratum; the filled gray dots (●) corresponded to the intermediate stratum; and the unfilled black dots (○) corresponded to the deep stratum
Discussion
Our hypothesis that the total richness and abundance (after exclusion of bdelloid rotifers) of invertebrate hatchlings decrease with sediment depth was supported in this study. Similarly, a range of studies on other wetland systems report greater abundance of hatchlings in the top layer of the sediment for several taxa, including Ostracoda, Cladocera and Copepoda, but mostly for rotifers (Carvalho and Wolf 1989; De Stasio 1990; Hairston et al. 1995; Ning and Nielsen 2011). Our results are in line with the idea that the surface layers of the sediment have the highest concentrations of viable dormant stages, responding better to hatching stimuli (Cáceres 1998; Cáceres and Hairston 1998; Hairston et al. 2000; Santangelo 2009). Another plausible explanation for hatching pattern observed may be related to the temporal degradation of the dormant eggs at deeper sediment depths that leads to higher mortality due to senescence, disease, and parasitism (Hairston et al. 1995, 2000; Kerfoot et al. 1999; Brendonck and De Meester 2003). Although it was not possible to date the sediment in our study, the positive relationship between the depth at which the invertebrate dormant stages are found in the sediment and their age is well known in undisturbed aquatic systems (Brendonck and De Meester 2003; Kerfoot and Weider 2004).
Studies that report the presence of dormant stages in southern Brazil wetlands only analyzed the top layers of the sediment (Palazzo et al. 2008; Ávila et al. 2015; Freiry et al. 2016; Stenert et al. 2016, 2017). This study evaluated the hatching of dormant stages of aquatic invertebrates across different depths of sediment (from top to deeper layers) in intermittent ponds, showing that most hatchings were from the Phylum Rotifera, Phylum Gatrotricha and Phylum Annelida. Some studies that evaluated only the top layers of the sediment in intermittent ponds of the same region (Freiry et al. 2020a, b; Vendramin et al. 2020, 2022) showed that the crustaceans from the Order Anomopoda (cladoceran species) were the most representative in the hatched invertebrate community. In our study, the hatchlings of the cladoceran species were also mainly related to the top layers of the sediment (5–6 cm).
The bdelloid rotifers represented by Adineta sp. and Philodina sp. comprised 64% of the total abundance found in this study. The high dominance of these two genera may be related to two factors: quick response from its dormant stages to environmental cues and asexual reproduction within 24 h. Rotifers of the Subclass Bdelloidea are known for their parthenogenesis and their dormant stage (anhydrobiosis), which allow them to withstand severe periods of desiccation (Ricci 2001). The physiological mechanisms that allow bdelloid rotifers to survive dehydrated during dormancy involve the protection of molecules such as sugars, proteins, and antioxidants (Rebecchi 2013), and the ability to recover their DNA when rehydrated (Hespeels et al. 2014). When water returns to the system, dormancy is broken, and within 24 h the individuals can reproduce by parthenogenesis (Ricci 2001). In this sense, although the sampling intervals of 2–3 days were used to minimize the chance of parthenogenetic reproduction (Brock et al. 2005; Nielsen et al. 2013), we cannot assume that all individuals of Bdelloidea found in this study are hatchlings from dormant stages.
In our experiment, taxa such as Aeolosomatidae, Nematoda and Gastrotricha hatched at depths greater than 20 cm. These taxa have specific dormancy characteristics, sheltering in the sediment until they finish the metabolic processes related to the dormant stages (Poinar Jr 2010; Strayer et al. 2010; Alekseev and Pinel-Alloul 2019; Fontaneto 2019). Aeolosomatidae can form desiccation-resistant cysts (hardened membrane of mucus secreted by the worm) that allow them to survive adverse environmental conditions (Glasby et al. 2021). Gastrotrichs produce resting eggs that are thick-shelled and very resistant to freezing and drying (Strayer et al. 2010). These organisms generally occur in the upper 5 cm of the sediment, but depending on environmental conditions, they may occur at greater depths (Ricci and Balsamo 2000). Nematodes can coil, losing most of their internal water and halting their metabolic activity, remaining in this dormant condition until water becomes available again (Rebecchi et al. 2007). There is evidence that the survival of adult dormant nematodes can be extremely long, reaching over 30 years (McSorley 2003).
Our result showed the presence of dormant stages capable of hatching in sediments up to 30 cm deep in intermittent wetlands. In ecological terms, this information is important, even if the stimuli necessary for hatching are reduced in deeper sediments. Dormant stages of deeper sediments can reach the surface through the stirring of the sediment by animals. Fish, insects, worms and wetland molluscs can commonly disturb sediments (Brendonck and De Meester 2003). Intermittent ponds are often visited by different species that, when interacting with the environment, can disturb the sediment and expose the dormant stages to the surface, such as watering cattle, birds and other large mammals (Brendonck and De Meester 2003). The reduction of dormant stages along the sediment depth of wetlands also is important in terms of conservation and restoration. The existence of dormant stages in the deepest parts of the sediment can be fundamental for the resilience of aquatic invertebrates when the dormant forms of the surface sediment are compromised with environmental impact.
A greater richness and abundance of invertebrate hatchlings were observed in the top layers of sediment (up to 10 cm). Our results demonstrate that the hatching rate of invertebrates decreases with depth in sediments from temporary wetlands. These results help to understand the dormancy breaking strategies of aquatic invertebrates that produce dormant stages in temporary wetlands, and they are important to understand the recovery capacity of dormant community from different sediment strata after drought events. As intermittent wetlands are extremely susceptible to climate variations, the results help to show the resilience of drought resistant communities in the face of unstable hydrological dynamics of these ecosystems.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
References
Alekseev VR, Pinel-Alloul B (2019) Dormancy in aquatic organisms. Theory, human use and modeling. Springer, Cham. https://doi.org/10.1007/978-3-030-21213-1
Anderson MJ (2006) Distance-based tests for homogeneity of multivariate dispersions. Biometrics 62:245–253. https://doi.org/10.1111/j.1541-0420.2005.00440.x
Angeler DG (2007) Resurrection ecology and global climate change research in freshwater ecosystems. J North Am Benthol Soc 26:12–22. https://doi.org/10.1899/0887-3593(2007)26[12:REAGCC]2.0.CO;2
Ávila AC, Boelter T, Santos RM, Stenert C, Würdig NL, Rocha O, Maltchik L (2015) The effects of different rice cultivation systems and ages on resting stages of wetland invertebrates in southern Brazil. Mar Freshw Res 66:276–285. https://doi.org/10.1071/MF14048
Brazil T, Caetano ACL, Vargas AL, Bozelli RL, Santangelo JM (2022) Desiccation increases the hatching of resting eggs of a freshwater calanoid copepod. J Plankton Res 44:273–277. https://doi.org/10.1093/plankt/fbac008
Brendonck L (1996) Diapause, quiescence, hatching requirements: what we can learn from large freshwater branchiopods (Crustacea: Branchiopoda: Anostraca, Notostraca, Conchostraca). Hydrobiologia 320:85–97. https://doi.org/10.1007/BF00016809
Brendonck L, De Meester L (2003) Egg banks in freshwater zooplankton: evolutionary and ecological archives in the sediment. Hydrobiologia 491:65–84. https://doi.org/10.1023/A:1024454905119
Brendonck L, Pinceel T, Ortells R (2017) Dormancy and dispersal as mediators of zooplankton population and community dynamics along a hydrological disturbance gradient in inland temporary pools. Hydrobiologia 796:201–222. https://doi.org/10.1007/s10750-016-3006-1
Brock MA, Nielsen DL, Shiel RJ, Green JD, Langley JD (2003) Drought and aquatic community resilience: the role of eggs and seeds in sediments of temporary wetlands. Freshw Biol 48:1207–1218. https://doi.org/10.1046/j.1365-2427.2003.01083.x
Brock MA, Nielsen DL, Crosslé K (2005) Changes in biotic communities developing from freshwater wetland sediments under experimental salinity and water regimes. Freshw Biol 50:1376–1390. https://doi.org/10.1111/j.1365-2427.2005.01408.x
Cáceres CE (1998) Interspecific variation in the abundance, production, and emergence of Daphnia diapausing eggs. Ecology 79:1699–1710. https://doi.org/10.2307/176789
Cáceres CE, Hairston NG JR (1998) Benthic-pelagic coupling in planktonic crustaceans: the role of the benthos. Arch Hydrobiol 52:163–174
Carvalho GR, Wolf HG (1989) Resting eggs of lake- Daphnia I. distribution, abundance and hatching of eggs collected from various depths in lake sediments. Freshw Biol 22:459–470. https://doi.org/10.1111/j.1365-2427.1989.tb01118.x
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Austral Ecol 18:117–143
Cousyn C, De Meester L (1998) The vertical profile of resting egg banks in natural populations of the pond-dwelling cladoceran Daphnia magna. Arch Hydrobiol 52:127–139
da Silva Bandeira MG, Martins KP, Palma-Silva C, Hepp LU, Albertoni EF (2020) Hydration time influences microcrustacean hatching in intermittent wetlands: in situ and ex situ approaches. Hydrobiologia 847:3227–3245. https://doi.org/10.1007/s10750-020-04315-w
Day J, Day E, Ross-Gillespie V, Ketley A (2010) The assessment of temporary wetlands during dry conditions.Water Research Commission Report TT434/09, Water Research Commission. https://www.wrc.org.za/wpcontent/uploads/mdocs/TT&2043409%20Conservation%20of%20Water%20Ecosystems.pdf
De Stasio BT Jr (1990) The role of dormancy and emergence patterns in the dynamics of a freshwater zooplankton community. Limnol Oceanogr 35:1079–1090. https://doi.org/10.4319/lo.1990.35.5.1079
Ellner S, Hairston NG Jr (1994) Role of overlapping generations in maintaining genetic variation in a fluctuating environment. Am Nat 143:403–417. https://doi.org/10.1086/285610
Elmoor-Loureiro LMA (1997) Manual de identificação de cladóceros límnicos do Brasil. Universa, Universidade Católica de Brasília, Brasília
Fontaneto D (2019) Long-distance passive dispersal in microscopic aquatic animals. Mov Ecol 7:1–10. https://doi.org/10.1186/s40462-019-0155-7
Freiry RF, Esquinatti FM, Stenert C, Arenzon A, Nielsen DL, Maltchik L (2016) Effects of spatial scale and habitat on the diversity of diapausing wetland invertebrates. Aquat Biol 25:173–181. https://doi.org/10.3354/ab00666
Freiry RF, Weber V, Bonecker CC, Lansac-Tôha FA, Pires MM, Stenert C, Maltchik L (2020a) Additive partitioning of the diversity of the dormant zooplankton communities in intermittent ponds along a forest–grassland transition. Hydrobiologia 847:1327–1342. https://doi.org/10.1007/s10750-020-04187-0
Freiry RF, Gouvea A, Becker J, Lansac-Tôha FA, Lansac-Tôha FM, Pires MM, Stenert C, Maltchik L (2020b) Community structure and concordance patterns among zooplankton life stages in subtropical temporary ponds. Aquat Ecol 54:257–270. https://doi.org/10.1007/s10452-019-09740-1
Frisch D, Morton PK, Chowdhury PR, Culver BW, Colbourne JK, Weider LJ, Jeyasingh PD (2014) A millennial-scale chronicle of evolutionary responses to cultural eutrophication in Daphnia. Ecol Lett 17:360–368. https://doi.org/10.1111/ele.12237
García-Roger EM, Carmona MJ, Serra M (2017) Modes, mechanisms and evidence of bet hedging in rotifer diapause traits. Hydrobiologia 796:223–233. https://doi.org/10.1007/s10750-016-2869-5
García-Roger EM, Ortells R (2018) Trade-offs in rotifer diapausing egg traits: survival, hatching, and lipid content. Hydrobiologia 805:339–350. https://doi.org/10.1007/s10750-017-3317-x
Gazulha V (2012) Zooplâncton límnico: manual ilustrado. Technical Books, Rio de Janeiro
Glasby CJ, Erséus C, Martin P (2021) Annelids in extreme aquatic environments: diversity, adaptations and evolution. Diversity 13:98. https://doi.org/10.3390/d13020098
Gyllström M, Hansson LA (2004) Dormancy in freshwater zooplankton: induction, termination and the importance of benthic-pelagic coupling. Aquat Sci 66:274–295. https://doi.org/10.1007/s00027-004-0712-y
Hairston NG Jr (1996) Zooplankton egg banks as biotic reservoirs in changing environments. Limnol Oceanogr 41:1087–1092. https://doi.org/10.4319/lo.1996.41.5.1087
Hairston NG Jr, Vanbrunt RA, Kearns CM, Engstrom DR (1995) Age and survivorship of diapausing eggs in a sediment egg bank. Ecology 76:1706–1711. https://doi.org/10.2307/1940704
Hairston NG Jr, Perry LJ, Bohonak AJ, Fellows MQ, Kearns CM, Engstrom DR (1999) Population biology of a failed invasion: paleolimnology of Daphnia exilis in upstate New York. Limnol Oceanogr 44:477–486. https://doi.org/10.4319/lo.1999.44.3.0477
Hairston NG Jr, Hansen AM, Schaffner WR (2000) The effect of diapause emergence on the seasonal dynamics of a zooplankton assemblage. Freshw Biol 45:133–145. https://doi.org/10.1046/j.1365-2427.2000.00386.x
Herzig A (1985) Resting eggs – a significant stage in the life cycle of crustaceans Leptodora kindti and Bythotrephes longimanus. Verh Int Ver Theor Angew Limnol 22:3088–3098. https://doi.org/10.1080/03680770.1983.11897838
Hespeels B, Knapen M, Hanot-Mambres D, Heuskin AC, Pineux F, Lucas S, Koszul R, Van Doninck K (2014) Gateway to genetic exchange? DNA double-strand breaks in the bdelloid rotifer Adineta vaga submitted to desiccation. J Evol Biol 27:1334–1345. https://doi.org/10.1111/jeb.12326
Iglesias C, Bonecker C, Brandão L, Crispim MC, Eskinazi-Sant’Anna EM, Gerhard M, Portinho JL, Maia-Barbosa P, Panarelli E, Santangelo JM (2016) Current knowledge of South American cladoceran diapause: a brief review. Int Rev Hydrobiol 101:91–104. https://doi.org/10.1002/iroh.201501825
Kerfoot WC, Robbins JA, Weider LJ (1999) A new approach to historical reconstruction: combining descriptive and experimental paleolimnology. Limnol Oceanogr 44:1232–1247. https://doi.org/10.4319/lo.1999.44.5.1232
Kerfoot WC, Weider LJ (2004) Experimental paleoecology (resurrection ecology): chasing Van Valen’s Red Queen hypothesis. Limnol Oceanogr 49:1300–1316
Koste W (1978) Rotatoria. Gebrüder Bosntraeget, Stuttgart
Maia-Barbosa PM, Eskinazi-Sant’Anna EM, Valadares CF, Pessoa GCD (2003) The resting eggs of zooplankton from a tropical, eutrophic reservoir (Pampulha Reservoir, south-east Brazil). Lakes Reserv Res Manag 8:269–275. https://doi.org/10.1111/j.1440-1770.2003.00229.x
Maltchik L, Costa ES, Becker CG, Oliveira AE (2003) Inventory of wetlands of Rio Grande do sul (Brazil). Pesq Bot 53:89–100
Marcus NH, Lutz R, Burnettt W, Cable P (1994) Age, viability, and vertical distribution of zzoplankton resting eggs from and anoxic basin: evidence of an egg bank. Limnol Oceanog 39:154–158. https://doi.org/10.4319/lo.1994.39.1.0154
McSorley R (2003) Adaptations of nematodes to environmental extremes. Fla Ent 86:138–142
Nielsen DL, Smith FJ, Hillman TJ, Shiel RJ (2000) Impact of water regime and fish predation on zooplankton resting egg production and emergence. J Plankton Res 22:433–446. https://doi.org/10.1093/plankt/22.3.433
Nielsen DL, Podnar K, Watts RJ, Wilson AL (2013) Empirical evidence linking increased hydrologic stability with decreased biotic diversity within wetlands. Hydrobiologia 708:81–96. https://doi.org/10.1007/s10750-011-0989-5
Ning NSP, Nielsen DL (2011) Community structure and composition of microfaunal egg bank assemblages in riverine and floodplain sediments. Hydrobiologia 661:211–221. https://doi.org/10.1007/s10750-010-0525-z
Palazzo F, Bonecker CC, Nagae MY (2008) Zooplankton dormancy forms in two environments of the upper Paraná River floodplain (Brazil). Acta Limnol Bras 20:55–62
Panarelli EA, Casanova SMC, Henry R (2008) The role of resting eggs in the recovery of zooplankton community in a marginal lake of the Paranapanema River (São Paulo, Brazil), after a long drought period. Acta Limnol Bras 20:73–88
Parra G, Guerrero F, Armengol J, Brendock L, Brucet S, Finlayson CM, Gomes-Barbosa L, Grillas P, Jeppesen E, Ortega F, Vega R, Zohary T (2021) The future of temporary wetlands in drylands under global change. Inland Waters 11:445–456. https://doi.org/10.1080/20442041.2021.1936865
Poinar GO Jr (2010) Nematoda and Nematomorpha. In: Thorp JH, Covich AP (eds) Ecology and classification of freshwater invertebrates of North America, 3rd edn. Academic Press, San Diego, pp 237–276
R Development Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rambo B (2000) A Fisionomia do Rio Grande do sul. Unisinos, São Leopoldo, Brazil
Rebecchi L (2013) Dry up and survive: the role of antioxidant defences in anhydrobiotic organisms. J Limnol 72:62–72. https://doi.org/10.4081/jlimnol.2013.s1.e8
Rebecchi L, Altiero T, Guidetti R (2007) Anhydrobiosis: the extreme limit of desiccation tolerance. Invert Surv J 4:65–81
Ricci C (2001) Dormancy patterns in rotifers. Hydrobiologia 446:1–11. https://doi.org/10.1023/A:1017548418201
Ricci C, Balsamo M (2000) The biology and ecology of lotic rotifers and gastrotrichs. Freshw Biol 44:15–28. https://doi.org/10.1046/j.1365-2427.2000.00584.x
Rogalski MA (2015) Tainted resurrection: metal pollution is linked with reduced hatching and high juvenile mortality in Daphnia egg banks. Ecology 96:1166–1173. https://doi.org/10.1890/14-1663.1
Rogalski MA, Leavitt PR, Skelly DK (2017) Daphniid zooplankton assemblage shifts in response to eutrophication and metal contamination during the Anthropocene. Proc R Soc B 284:1471–2954. https://doi.org/10.1098/rspb.2017.0865
Santangelo JM (2009) Produção, eclosão e implicações ecológicas e evolutivas dos estágios dormentes do zooplâncton. Limnotemas. Sociedade Brasileira de Limnologia, Rio de Janeiro
Santangelo JM, Esteves FA, Manca M, Bozelli RL (2014) Disturbances due to increased salinity and the resilience of zooplankton communities: the potential role of the resting egg bank. Hydrobiologia 722:103–113. https://doi.org/10.1007/s10750-013-1683-6
Shmakova L, Malavin S, Iakovenko N, Vishnivetskaya T, Shain D, Plewka M, Rivkina E (2021) A living bdelloid rotifer from 24,000-year-old Arctic permafrost. Curr Biol 31:R712–R713. https://doi.org/10.1016/j.cub.2021.04.077
Sieger R, Grobe H (2013) Panplot 2: software to visualize profiles and time series. Helmholtz Centre for Polar and Marine Research, Bremerhaven, PANGAEA. Alfred Wegner Institute. https://doi.org/10.1594/PANGAEA.816201
Stenert C, Bacca RC, Ávila AC, Maltchik L, Rocha O (2010) Do hydrologic regimes used in rice fields compromise the viability of resting stages of aquatic invertebrates? Wetlands 30:989–996. https://doi.org/10.1007/s13157-010-0083-1
Stenert C, Ehlert B, Ávila AC, Sousa FDR, Esquinatti FM, Batzer DP, Maltchik L (2016) Dormant propagule banks of aquatic invertebrates in ponds invaded by exotic pine species in southern Brazil. Mar Freshw Res 68:954–963. https://doi.org/10.1071/MF16067
Stenert C, Wüsth R, Pires MM, Freiry RF, Nielsen D, Maltchik L (2017) Composition of cladoceran dormant stages in intermittent ponds with different hydroperiod lengths. Ecol Res 32:921–930. https://doi.org/10.1007/s11284-017-1498-4
Strachan SR, Chester ET, Robson BJ (2015) Freshwater invertebrate life history strategies for surviving desiccation. Springer Sci Rev 3:57–75. https://doi.org/10.1007/s40362-015-0031-9
Strayer DL, Hummon WD, Hochberg R (2010) Gastrotricha. In: Thorp JH, Covich AP (eds) Ecology and classification of freshwater invertebrates of North America, 3rd edn. Academic Press, San Diego, pp 163–172
Vanschoenwinkel B, Seaman M, Brendonck L (2010) Hatching phenology, life history and egg bank size of fairy shrimp Branchipodopsis spp. (Branchiopoda, Crustacea) in relation to the ephemerality of their rock pool habitat. Aquat Ecol 44:771–780. https://doi.org/10.1007/s10452-010-9315-y
Vargas AL, Santangelo JM, Bozelli RL (2019) Recovery from drought: viability and hatching patterns of hydrated and desiccated zooplankton resting eggs. Internat Rev Hydrobiol 104:26–33. https://doi.org/10.1002/iroh.201801977
Vendramin D, Klagenberg CS, Provensi MR, Stenert C, Pires MM, Medeiros ESF, Reichard M, Maltchik L (2020) Effects of the presence of annual killifish on the assemblage structure of resting stages of aquatic invertebrates in temporary ponds. Limnetica 39:1–16. https://doi.org/10.23818/limn.39.01
Vendramin D, Pires MM, Freiry RF, Schneider AEB, Martins L, Medeiros ESF, Rocha O, Stenert C, Maltchik L (2022) Hatching dynamics of invertebrate dormant stages in temporary ponds are influenced by multiple hydrations. Freshw Sci 41:143–152. https://doi.org/10.1092/23F2-2XBW-252B-DU5C
Wang CC, Chou LS (2015) Terminating dormancy: hatching phenology of sympatric large branchiopods in Siangtian Pond, a temporary wetland in Taiwan. J Crustac Biol 35:301–308. https://doi.org/10.1163/1937240X-00002322
Williams DD (2006) The biology of temporary waters. Oxford University Press, New York
Yousey AM, Chowdhury PR, Biddinger N, Shaw JH, Jeyasing PD, Weider LJ (2018) Resurrected ‘ancient’ Daphnia genotypes show reduced thermal stress tolerance compared to modern descendants. R Soc Open Sci 5:2054–5703. https://doi.org/10.1098/rsos.172193
Acknowledgements
We thank Gerson Fauth, Simone Fauth and Guilherme Krahl for their scientific support and the sample equipment.
Author information
Authors and Affiliations
Contributions
Pedro Henrique de Oliveira Hoffmann, Cristina Stenert and Leonardo Maltchik contributed to the study conception, design and writing – first draft, review and editing. Field and laboratory work were performed by Allana Gonçalves Piu, Lidiane Martins, Vinicius Weber and Daiane Vendramin. Data analysis were performed by Cristina Stenert, Pedro Henrique de Oliveira Hoffmann and Andressa Adolfo. Funding acquisition, project administration and supervision were peformed by Cristina Stenert and Leonardo Maltchik. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Statements and Declarations
Funding.
This research was supported by CNPq - Conselho Nacional de Desenvolvimento Científico e Tecnológico (Grant number 474892/2013-1). CNPq granted IC scholarships to PHOH, AGP, LM, VW. CAPES supported DV with a doctoral fellowship. LM and CS hold Research Productivity Grants from CNPq.
Conflict of interest/Competing Interests
The authors declare no conflict of interest regarding this publication. The authors have no relevant financial or non-financial interests to disclose.
Ethics Approval
We declare that data collection complied with the current Brazilian environmental laws (SISBIO 36365-2).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Oliveira Hoffmann¹, P.H., Adolfo², A., Piu², A.G. et al. Invertebrate Richness and Hatching Decrease with Sediment Depth in Neotropical Intermittent Ponds. Wetlands 43, 24 (2023). https://doi.org/10.1007/s13157-023-01675-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13157-023-01675-6