Abstract
Purpose
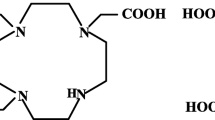
The present paper reports a systematic study on the effect of bifunctional chelators (BFC) namely, NOTA, DOTA, and DTPA, on the radiochemical formulation, in vitro stability, and in vivo biological properties of 68Ga-labeled RGD peptide derivatives.
Methods
The three RGD conjugates namely, NOTA-Bn-E-[c(RGDfk)]2, DOTA-Bn-E-[c(RGDfk)]2, and DTPA-Bn-E-[c(RGDfk)]2 were radiolabeled with 68Ga and the radiolabeling was optimized with respect to the ligand amount, radiolabeling time, and temperature. Further, the 68Ga complexes were assessed for their in vitro and in vivo stabilities. The biodistribution studies of the three radiolabeled conjugates were carried out in C57BL/6 mice bearing melanoma tumor at 30 min and 1 h post-adimistration.
Results
NOTA-Bn-E-[c(RGDfk)]2 could be radiolabeled with 68Ga at room temperature while DOTA-Bn-E-[c(RGDfk)]2 and DTPA-Bn-E-[c(RGDfk)]2 were radiolabeled at high temperature. 68Ga-NOTA-Bn-E-[c(RGDfk)]2 was found to be the most kinetically rigid in in vitro stability assay. The uptake of the three radiolabeled peptide conjugates in melanoma tumor was comparable at 1 h post-administration (NOTA; DOTA; DTPA (% I.D./g):: 2.78 ± 0.38; 3.08 ± 1.1; 3.36 ± 0.49). However, the tumor/background ratio of 68Ga-NOTA-Bn-E-[c(RGDfk)]2 was the best amongst the three radiotracers. 68Ga-complexes of NOTA-Bn-E-[c(RGDfk)]2 and DOTA-Bn-E-[c(RGDfk)]2 showed excellent in vivo stability while 68Ga-DTPA-Bn-E-[c(RGDfk)]2 showed significant metabolic degradation.
Conclusion
These studies show that 68Ga-NOTA-Bn-E-[c(RGDfk)]2 would be the most appropriate 68Ga-labeled radiotracer and the most amenable for kit formulation.







Similar content being viewed by others
References
Backer MV, Backer JM. Imaging key biomarkers of tumor angiogenesis. Theranostics. 2012;2:502–15.
Cai W, Chen X. Multimodality molecular imaging of tumor angiogenesis. J Nucl Med. 2008;49:113S–28S.
Chakravarty R, Chakraborty S, Dash A. Molecular imaging of breast cancer: role of RGD peptides. Mini-Rev Med Chem. 2015;15:1073–94.
Danhier F, Le Breton A, Preat V. RGD-based strategies to target alpha(v) beta(3) integrin in cancer therapy and diagnosis. Mol Pharm. 2012;9:2961–73.
Weis SM, Cheresh DA. New approaches to image angiogenesis. Nat Med. 2011;17:1359–70.
Cai H, Conti PS. RGD-based PET tracers for imaging receptor integrin alphav beta3 expression. J Labelled Comp Radiopharm. 2013;56:264–79.
Haubner R, Maschauer S, Prante O. PET radiopharmaceuticals for imaging integrin expression: tracers in clinical studies and recent developments. Bio Med Res Int. 2014;871609:1–17.
Liu S. Radiolabeled cyclic RGD peptides as integrin αvβ3-targeted radiotracers: maximizing binding affinity via bivalency. Bioconjug Chem. 2009;20:2199–213.
Liu S. Radiolabeled multimeric cyclic RGD peptides as integrin αvβ3 targeted radiotracers for tumor imaging. Mol Pharm. 2006;3:472–87.
Niu G, Chen X. PET imaging of angiogenesis. PET Clin. 2009;4:17–38.
Tateishi U, Oka T, Inoue T. Radiolabeled RGD peptides as integrin αvβ3-targeted PET tracers. Curr Med Chem. 2012;19:3301–9.
Ziegler SI. Positron emission tomography: principles, technology, and recent developments. Nucl Phys A. 2005;752:679–87.
Iagaru A, Mosci C, Shen B, Chin FT, Mittra E, Telli ML, et al. 18F-FPPRGD2 PET/CT: pilot phase evaluation of breast cancer patients. Radiology. 2014;273:549–59.
Kenny LM, Coombe RC, Oulie I, Contractor KB, Miller M, Spinks TJ, et al. Phase I trial of the positron-emitting Arg-Gly-Asp (RGD) peptide radioligand 18F-AH111585 in breast cancer patients. J Nucl Med. 2008;49:879–86.
Röesch F, Riss PJ. The renaissance of the 68Ge/68Ga radionuclide generator initiates new developments in 68Ga radiopharmaceutical chemistry. Curr Top Med Chem. 2010;10:1633–68.
Goodwin DA, Ransone CM, Diamanti CI, McTigue M. Rapid synthesis and quality control of 68Ga-labeled chelates for clinical use. Nucl Med Biol. 1994;21:897–9.
Prata MI, Santos AC, Geraldes CF, De Lima JJ. Structural and in vivo studies of metal chelates of Ga(III) relevant to biomedical imaging. J Inorg Biochem. 2000;79:359–63.
Velikyan I, Maeck H, Langstrom B. Convenient preparation of 68Ga based PET radiopharmaceuticals at room temperature. Bioconjug Chem 2008; 19:569–573.
Chakraborty S, Chakravarty R, Vatsa R, Bhusari P, Sarma HD, Shukla J, et al. Toward realization of ‘mix-and-use’ approach in 68Ga radiopharmacy: preparation, evaluation and preliminary clinical utilization of 68Ga-labeled NODAGA-coupled RGD peptide derivative. Nucl Med Biol. 2016;43:116–23.
Dijkgraaf I, Yim CB, Franssen GM, Schuit RC, Luurtsema G, Liu S, et al. PET imaging of αvβ3 integrin expression in tumours with 68Ga-labelled mono-, di- and tetrameric RGD peptides. Eur J Nucl Med Mol Imaging. 2011;38:128–37.
Knetsch PA, Petrik M, Griessinger CM, Rangger C, Fani M, Kesenheimer C, et al. [68Ga]NODAGA-RGD for imaging alphavbeta3 integrin expression. Eur J Nucl Med Mol Imaging. 2011;38:1303–12.
Li ZB, Chen K, Chen X. 68Ga-labeled multimeric RGD peptides for microPET imaging of integrin alpha(v)beta (3) expression. Eur J Nucl Med Mol Imaging. 2008;35:1100–8.
Lopez-Rodriguez V, Gaspar-Carcamo RE, Pedraza-Lopez M, Rojas-Calderon EL, Arteaga de Murphy C, Ferro-Flores G, et al. Preparation and preclinical evaluation of 66Ga-DOTA-E(c(RGDfK))2 as a potential theranostic radiopharmaceutical. Nucl Med Biol. 2015;42:109–14.
Notni J, Pohle K, Wester HJ. Comparative gallium-68 labeling of TRAP-, NOTA-, and DOTA-peptides: practical consequences for the future of gallium-68-PET. EJNMMI Res. 2012;2:28.
Oxboel J, Brandt-Larsen M, Schjoeth-Eskesen C, Myschetzky R, El-Ali HH, Madsen J, et al. Comparison of two new angiogenesis PET tracers 68Ga-NODAGA-E[c(RGDyK)]2 and 64Cu-NODAGA-E[c(RGDyK)]2; in vivo imaging studies in human xenograft tumors. Nucl Med Biol. 2014;41:259–67.
Pohle K, Notni J, Bussemer J, Kessler H, Schwaiger M, Beer AJ. 68Ga-NODAGA-RGD is a suitable substitute for 18F-Galacto-RGD and can be produced with high specific activity in a cGMP/GRP compliant automated process. Nucl Med Biol. 2012;39:777–84.
Ferreira CL, Donald TYY, Mandel D, Gill KR, Boros E, Wong MQ, et al. 68Ga small peptide imaging: comparison of NOTA and PCTA. Bioconjug Chem. 2012;23:2239–46.
Chakravarty R, Chakraborty S, Dash A, Pillai MRA. Detailed evaluation on the effect of metal ion impurities on complexation of generator eluted 68Ga with different bifunctional chelators. Nucl Med Biol. 2013;40:197–205.
Acknowledgments
The authors are thankful to Dr. Aruna Korde, Head, Radiopharmaceutical Evaluation Section, Radiopharmaceuticals Division, BARC for providing access to the 68Ge/68Ga generator. Thanks are also due to Dr. B.S. Tomar, Director, Radiochemistry & Isotope Group, BARC for his support and encouragement.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
Akanksha Jain, Sudipta Chakraborty, Haladhar Dev Sarma and Ashutosh Dash, declare that they have no conflict of interest financial, scientific or otherwise in the publication of this article. Research at the Bhabha Atomic Research Centre (BARC) is part of the ongoing activities of the Department of Atomic Energy, India and is fully supported by government funding.
Ethical Approval
All procedures performed in studies involving animals were in strict compliance with the approved protocols of Institutional Animal Ethics Committee of BARC, India.
Informed Consent
The institutional review board of our institute, BARC, approved this retrospective study, and the requirement to obtain informed consent was waived.
Rights and permissions
About this article
Cite this article
Jain, A., Chakraborty, S., Sarma, H.D. et al. A Systematic Comparative Evaluation of 68Ga-Labeled RGD Peptides Conjugated with Different Chelators. Nucl Med Mol Imaging 52, 125–134 (2018). https://doi.org/10.1007/s13139-017-0499-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13139-017-0499-0