Abstract
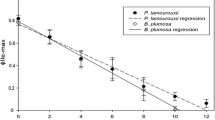
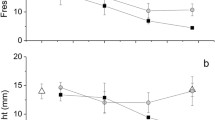
Solar-powered sea slugs (Sacoglossa: Gastropoda) have long captured the attention of laymen and scientists alike due to their remarkable ability to steal functional chloroplasts from their algal food, enslaving them to withstand long starvation periods. Recently, a wealth of data has shed insight into this remarkable relationship; however, the cellular mechanisms governing this process are still completely unknown. This study explores these mechanisms, providing insight into the chloroplast retention and delayed digestion, occurring within the slug’s digestive gland. We examine the relationships between functional chloroplast and lysosome abundances during starvation, in live material, for the long-term retaining species Elysia timida, the ambiguous long/short-term retaining Elysia viridis, and the short-term retaining Thuridilla hopei, to elucidate digestive differences that contribute to the development of functional kleptoplasty. Functional chloroplast and lysosome abundance are measured using chlorophyll a autofluorescence and the pH-dependent stain acridine orange. In each species, the number of chloroplasts and lysosomes is indirectly proportional, with the plastid density decreasing when starvation begins. We also present a new FIJI/Image J Plugin, the 3D—Accounting and Measuring Plugin, 3D-AMP, which enables the reliable analysis of large image sets.









Similar content being viewed by others
References
Baumgartner, F. A., Pavia, H., & Toth, G. B. (2015). Acquired phototrophy through retention of functional chloroplasts increases growth efficiency of the sea slug Elysia viridis. PLoS ONE, 10(4), e0120874. doi:10.1371%2Fjournal.pone.0120874.
Christa, G. (2014). Functional kleptoplasty in a limapontiodean genus: phylogeny, food preferences, and photosynthesis in costasiella with a focus on C. ocellifera (Gastropoda: Sacoglossa). Journal of Molluscan Studies.
Christa, G., Wescott, L., Schäberle, T. F., König, G. M., & Wägele, H. (2013a). What remains after 2 months of starvation? Analysis of sequestered algae in a photosynthetic slug, Plakobranchus ocellatus (Sacoglossa, Opisthobranchia), by barcoding. Planta, 237(2), 559–572. doi:10.1007/s00425-012-1788-6.
Christa, G., Zimorski, V., Woehle, C., Tielens, A. G. M., Wägele, H., Martin, W. F., & Gould, S. B. (2013b). Plastid-bearing sea slugs fix CO2 in the light but do not require photosynthesis to survive. Proceedings. Biological sciences / The Royal Society, 281(1774), 20132493. http://www.ncbi.nlm.nih.gov/pubmed/24258718.
Christa, G., Händeler, K., Kück, P., Vleugels, M., Franken, J., Karmeinski, D., & Wägele, H. (2014a). Phylogenetic evidence for multiple independent origins of functional kleptoplasty in Sacoglossa (Heterobranchia, Gastropoda). Organisms Diversity & Evolution, 15(1), 23–36.
Christa, G., Händeler, K., Schäberle, T. F., König, G. M., & Wägele, H. (2014b). Identification of sequestered chloroplasts in photosynthetic and non-photosynthetic sacoglossan sea slugs (Mollusca, Gastropoda). Frontiers in Zoology, 11(5).
Curtis, N. E., Massey, S. E., & Pierce, S. K. (2006). The symbiotic chloroplasts in the sacoglossan Elysia clarki are from several algal species. Invertebrate Biology, 125(4), 336–345.
Curtis, N. E., Middlebrooks, M. L., Schwartz, J. A., & Pierce, S. K. (2015). Kleptoplastic sacoglossan species have very different capacities for plastid maintenance despite utilizing the same algal donors. Symbiosis, 65(1), 23–31.
De Duve, C. (1963). The lysosome concept. In Ciba Foundation Symposium-Lysosomes (pp. 1–35). Wiley Online Library.
de Vries, J., Christa, G., Gould, SB. (2014). Plastid survival in the cytosol of animal cells. Trends in Plant Science 19(6):347–50. doi:10.1016/j.tplants.2014.03.010.
de Vries, J., Woehle, C., Christa, G., Wägele, H., Tielens, A. G. M., Jahns, P., & Gould, S. B. (2015). Comparison of sister species identifies factors underpinning plastid compatibility in green sea slugs. Proceedings of the Royal Society of London B: Biological Sciences, 282(1802). http://rspb.royalsocietypublishing.org/content/282/1802/20142519.abstract.
Driessche, T. V. (1966). Circadian rhythms in Acetabularia: photosynthetic capacity and chloroplast shape. Experimental Cell Research, 42(1), 18–30.
Gallop, A., Bartrop, J., & Smith, D. C. (1980). The biology of chloroplast acquisition by Elysia viridis. Proceedings of the Royal Society of London. Series B. Biological Sciences, 207(1168), 335–349.
Greene, R. W. (1970). Symbiosis in sacoglossan opisthobranchs: functional capacity of symbiotic chloroplasts. Marine Biology, 7(2), 138–142. doi:10.1007/BF00354917.
Händeler, K., Grzymbowski, Y. P., Krug, P. J., & Wägele, H. (2009). Functional chloroplasts in metazoan cells—a unique evolutionary strategy in animal life. Frontiers in Zoology, 6(1), 28.
Johnson, M. D. (2011). The acquisition of phototrophy: adaptive strategies of hosting endosymbionts and organelles. Photosynthesis Research, 107(1), 117–132.
Kusuzaki, K., Matsubara, T., Satonaka, H., Matsumine, A., Nakamura, T., Sudo, A., et al. (2014). Intraoperative Photodynamic Surgery (iPDS) with acridine orange for musculoskeletal sarcomas. Cureus, 6(9).
Lindholm, T., & Mork, A.-C. (1989). Symbiotic algae and plastids in planktonic ciliates. Memoranda Societatis pro Fauna et Flora Fennica, 65(1), 17–22.
Martin, R., Walther, P., & Tomaschko, K.-H. (2013). Phagocytosis of algal chloroplasts by digestive gland cells in the photosynthesis-capable slug Elysia timida (Mollusca, Opisthobranchia, Sacoglossa). Zoomorphology, 132(3), 253–259. doi:10.1007/s00435-012-0184-x.
McLean, N. (1976). Phagocytosis of chloroplasts in Placida dendritica (Gastropoda: Sacoglossa). Journal of Experimental Zoology, 197(3), 321–329.
Moriyama, Y., Takano, T., & Ohkuma, S. (1982). Acridine orange as a fluorescent probe for lysosomal proton pump. Journal of Biochemistry, 92(4), 1333–1336. http://jb.oxfordjournals.org/content/92/4/1333.abstract.
Pelletreau, K. N., Weber, A. P. M., Weber, K. L., & Rumpho, M. E. (2014). Lipid accumulation during the establishment of kleptoplasty in Elysia chlorotica. PLoS ONE, 9(5), e97477. doi:10.1371/journal.pone.0097477.
Rauch, C., de Vries, J., Rommel, S., Rose, L. E., Woehle, C., Christa, G., et al. (2015). Why it is time to look beyond algal genes in photosynthetic slugs. Genome Biology and Evolution, 7(9), 2602–2607.
Rumpho, M. E., Summer, E. J., & Manhart, J. R. (2000). Solar-powered sea slugs. Mollusc/algal chloroplast symbiosis. Plant Physiology, 123(1), 29–38.
Schmitt, V. (2011). Behavioral adaptations in relation to long-term retention of endosymbiotic chloroplasts in the Sea Slug Elysia timida. Thalassas, 27(2), 225–238.
Schmitt, V., Händeler, K., Gunkel, S., Escande, M.-L., Menzel, D., Gould, S. B., et al. (2014). Chloroplast incorporation and long-term photosynthetic performance through the life cycle in laboratory cultures of Elysia timida (Sacoglossa, Heterobranchia). Frontiers in Zoology, 11(1), 5. doi:10.1186/1742-9994-11-5.
Stoecker, D. K., Johnson, M. D., de Vargas, C., & Not, F. (2009). Acquired phototrophy in aquatic protists.
Taylor, D. L. (1968). Chloroplasts as symbiotic organelles in the digestive gland of Elysia viridis [Gastropoda: opisthobranchia]. Journal of the Marine Biological Association of the United Kingdom, 48(01), 1–15. doi:10.1017/S0025315400032380.
Ventura, P., Calado, G., & Jesus, B. (2013). Photosynthetic efficiency and kleptoplast pigment diversity in the sea slug Thuridilla hopei (Vérany, 1853). Journal of Experimental Marine Biology and Ecology, 441, 105–109. doi:10.1016/j.jembe.2013.01.022.
Wägele, H., & Johnsen, G. (2001). Observations on the histology and photosynthetic performance of “solar-powered” opisthobranchs (Mollusca, Gastropoda, Opisthobranchia) containing symbiotic chloroplasts or zooxanthellae. Organisms Diversity & Evolution, 1(3), 193–210. doi:10.1078/1439-6092-00016.
Wägele, H., & Martin, W. (2014). Endosymbioses in sacoglossan seaslugs: plastid-bearing animals that keep photosynthetic organelles without borrowing genes. In W. Löffelhardt (Ed.), Endosymbiosis SE - 14 (pp. 291–324). Vienna: Springer. doi:10.1007/978-3-7091-1303-5_14.
Wägele, H., Deusch, O., Händeler, K., Martin, R., Schmitt, V., Christa, G., et al. (2011). Transcriptomic evidence that longevity of acquired plastids in the photosynthetic slugs Elysia timida and Plakobranchus ocellatus does not entail lateral transfer of algal nuclear genes. Molecular Biology and Evolution, 28(1), 699–706. doi:10.1093/molbev/msq239.
Acknowledgments
We would like to thank Claudia Müller, Jörn van Döhren, Ekin Tilic, Daria Krämer, Albert Haas, Gregor Christa, Cessa Rauch, Jan de Vries, and Sven Gould and Ulf Bickmeyer for their expertise and help in this project. We also appreciate the feedback we received from our two anonymous reviewers.
This study was funded by the Deutsche Forschungsgemeinschaft project Wa 618/17, the Alexander Koenig Gesellschaft, and a personal grant to EMJL from the University of Bonn, Germany.
Author contributions
EMJL and HW devised the project concept and EMJL conducted the lab work. PTR and EMJL designed 3D-AMP and PTR wrote this software. AP and TB help troubleshoot staining and all authors contributed to the analysis and manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Laetz, E.M.J., Rühr, P.T., Bartolomaeus, T. et al. Examining the retention of functional kleptoplasts and digestive activity in sacoglossan sea slugs. Org Divers Evol 17, 87–99 (2017). https://doi.org/10.1007/s13127-016-0308-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-016-0308-0