Abstract
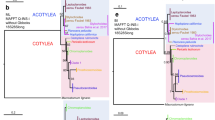
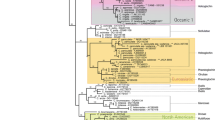
Annelid chaetae are a superior diagnostic character on species and supraspecific levels, because of their structural variety and taxon specificity. A certain chaetal type, once evolved, must be passed on to descendants, to become characteristic for supraspecific taxa. Therefore, one would expect that chaetal diversity increases within a monophyletic group and that additional chaetae types largely result from transformation of plesiomorphic chaetae. In order to test these hypotheses and to explain potential losses of diversity, we take up a systematic approach in this paper and investigate chaetation in Eunicida. As a backbone for our analysis, we used a three-gene (COI, 16S, 18S) molecular phylogeny of the studied eunicidan species. This phylogeny largely corresponds to previous assessments of the phylogeny of Eunicida. Presence or absence of chaetal types was coded for each species included into the molecular analysis and transformations for these characters were then estimated using the mK1 likelihood model. Our results show that chaetal type diversity does indeed increase within eunicids and provide possible explanations for the homology, convergence, and loss of chaetal types in eunicidan subtaxa.








Similar content being viewed by others
References
Aguado, M. T., Nygren, A., & Rouse, G. W. (2013). Two apparently unrelated groups of symbiotic annelids, Nautiliniellidae and Calamyzidae (Phyllodocida, Annelida), are a clade of derived chrysopetalid polychaetes. Cladistics, 29, 610–628.
Andrade, S. C. S., Novo, M., Kawauchi, G. Y., Worsaae, K., Pleijel, F., Giribet, G., & Rouse, G. W. (2015). Articulating “archiannelids”: phylogenomics and annelid relationships, with emphasis on meiofaunal taxa. Molecular Biology and Evolution. doi:10.1093/molbev/msv157.
Bartolomaeus, T. (1998). Chaetogenesis in polychaetous Annelida: significance for annelid systematics and the position of the Pogonophora. Zoology, 100, 348–364.
Bouligand, Y. (1967). Les soies et les cellules associées chez deux Annélides Polychètes. Zeitschrift für Zellforschung und mikroskopische Anatomie, 79(3), 332–363.
Carrera-Parra, L. (2006). Revision of Lumbrineris de Blainville, 1828 (Polychaeta: Lumbrineridae). Zootaxa, 1336, 1–64.
Caullery, M., & Mesnil, F. (1898). Les formes épitoques et l'évolution des cirratuliens. Annales de l'Université de Lyon, 39, 1–200.
Fauchald, K. (1977). The polychaete worms: definitions and keys to the orders, families and genera. Los Angeles: Natural History Museum of Los Angeles County.
Fauchald, K. (1982). Revision of onuphis, nothria, and paradiopatra (Polychaeta: Onuphidae) based upon type material. Washington DC: Smithsonian Institution Press.
Fauchald, K. (1992). A review of the genus Eunice (Polychaeta: Eunicidae) based upon type material. Washington, DC: Smithsonian Institution Press.
Gustus, R. M., & Cloney, R. A. (1973). Ultrastructure of the larval compound setae of the polychaete Nereis vexillosa Grube. Journal of Morphology, 140, 355–366.
Hartman, O. (1968). Atlas of the errantiate polychaetous annelids from California. Los Angeles: Allan Hancock Foundation, University of Southern California Press.
Hartman, O., & Fauchald, K. (1971). Deep-water benthic polychaetes off New England to Bermuda and other North Atlantic areas. Allan Hancock Monographs in Marine Biology, 6, 1–327.
Hartmann-Schröder, G., Dahl, F., & Schumann, H. (1996). Teil: Annelida- Borstenwürmer - Polychaeta (Vol. 58). Jena: Gustav Fischer Verlag.
Hausam, B., & Bartolomaeus, T. (2001). Ultrastructure and development of forked and capillary setae in the polychaetes Orbinia bioreti and Orbinia latreillii (Annelida: Orbiniidae). Invertebrate Biology, 120(1), 13–28.
Hausen, H. (2005). Chaetae and chaetogenesis in polychaetes (Annelida). Hydrobiologia, 535–536(1), 37–52.
Jumars, P. A. (1974). A generic revision of the Dorvilleidae (Polychaeta), with six new species from the deep North Pacific. Zoological Journal of the Linnean Society, 54(March), 101–135.
Katoh, K., Misawa, K., Kuma, K., & Miyata, T. (2002). MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research, 30(14), 3059–3066.
Lewis, P. O. (2001). A likelihood approach to estimating phylogeny from discrete morphological character data. Systematic Biology, 50(6), 913–925.
Maddison, W. P., & Maddison, D. R. (2015). Mesquite: a modular system for evolutionary analysis. http://mesquiteproject.org.
Merz, R. A. (2015). Textures and traction: how tube-dwelling polychaetes get a leg up. Invertebrate Biology, 134(1), 61–77.
Merz, R. A., & Edwards, D. R. (1998). Jointed setae—their role in locomotion and gait transitions in polychaete worms. Journal of Experimental Marine Biology and Ecology, 228, 273–290.
Merz, R. A., & Woodin, S. A. (2000). Hooked setae: tests of the anchor hypothesis. Invertebrate Biology, 19, 67–82.
Merz, R. A., & Woodin, S. (2006). Polychaete chaetae: function, fossils, and phylogeny. Integrative and Comparative Biology, 46(4), 481–496.
Nation, J. L. (1983). A new method using hexamethyldisilazane for preparation of soft insect tissues for scanning electron microscopy. Biotechnic & Histochemistry, 58(6), 347–351.
O’Clair, R., & Cloney, R. (1974). Patterns of morphogenesis mediated by dynamic microvilli: chaetogenesis in Nereis vexillosa. Cell and Tissue Research, 151(2), 141–157.
Okuda, S. (1946). Studies on the Development of Annelida Polychaeta I (With 17 Plates and 33 Textfigures). Journal of the Faculty of Science Hokkaido Imperial University Series VI. Zoology, 9(2), 115–219.
Osborn, K. J., & Rouse, G. W. (2011). Phylogenetics of Acrocirridae and Flabelligeridae (Cirratuliformia, Annelida). Zoologica Scripta, 40(2), 204–219.
Paxton, H. (1998). The Diopatra chiliensis confusion—redescription of D. chiliensis (Polychaeta, Onuphidae) and implicated species. Zoologica Scripta, 27(1), 31–48.
Pernet, B. (2000). A scaleworm’s setal snorkel. Invertebrate Biology, 119(2), 147–151.
Purschke, G. (1987). Anatomy and ultrastructure of ventral pharyngeal organs and their phylogenetic importance in Polychaeta (Annelida). IV. The pharynx and jaws of the Dorvilleidae. Acta Zoologica, 68(2), 83–105.
Rouse, G. W., & Fauchald, K. (1997). Cladistics and polychaetes. Zoologica Scripta, 26(2), 139–204.
Rouse, G. W., & Pleijel, F. (2001). Polychaetes. Oxford, New York: Oxford University Press.
Schroeder, P. (1984). Chaetae. In J. Bereiter-Hahn, A. G. Matoltsy, & K. S. Richards (Eds.), Biology of the integument (pp. 297–309). Berlin: Springer.
Silvestro, D., & Michalak, I. (2012). raxmlGUI: a graphical front-end for RAxML. Organisms Diversity & Evolution, 12(4), 335–337.
Specht, A., & Westheide, W. (1988). Intra-and interspecific ultrastructural character variation: the chaetation of the Microphthalmus listensis species group (Polychaeta, Hesionidae). Zoomorphology, 107, 371–376.
Stamatakis, A. (2006). RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics, 22(21), 2688–2690.
Struck, T. H., Purschke, G., & Halanych, K. M. (2006). Phylogeny of Eunicida (Annelida) and exploring data congruence using a partition addition bootstrap alteration (PABA) approach. Systematic Biology, 55(1), 1–20.
Struck, T. H., Golombek, A., Weigert, A., Franke, F. A., Westheide, W., & Purschke, G., et al. (2015). The evolution of annelids reveals two adaptive routes to the interstitial realm. Current Biology, 25(15), 1993–1999.
Swofford, D. L. (2002). PAUP* version 4.0. Phylogenetic analysis using parsimony (and other methods). Sinauer Associates, Sunderland, MA.
Tilic, E., Hausen, H., & Bartolomaeus, T. (2014). Chaetal arrangement and chaetogenesis of hooded hooks in Lumbrineris (Scoletoma) fragilis and Lumbrineris tetraura (Eunicida, Annelida). Invertebrate Biology, 133(4), 354–370.
Woodin, S. A., & Merz, R. A. (1987). Holding on by their hooks: anchors for worms. Evolution, 41(2), 427–432.
Worsaae, K. (2005). Phylogeny of Nerillidae (Polychaeta, Annelida) as inferred from combined 18S rDNA and morphological data. Cladistics, 21, 143–162. doi:10.1111/j.1096-0031.2005.00058.x.
Worsaae, K. (2014) Nerillidae Levinsen, 1883. In Westheide, W., & Purschke, G. (Eds.), Annelida polychaetes, handbook of zoology online. De Gruyter. http://www.degruyter.com/view/Zoology/bp_029147-6-10. Accessed 16 Nov 2015.
Worsaae, K., Nygren, A., Rouse, G. W., et al. (2005). Phylogenetic position of Nerillidae and Aberranta (Polychaeta, Annelida), analysed by direct optimization of combined molecular and morphological data. Zoolgica Scripta, 34, 313–328. doi:10.1111/j.1463-6409.2005.00190.x.
Zanol, J., Halanych, K. M., Struck, T. H., & Fauchald, K. (2010). Phylogeny of the bristle worm family Eunicidae (Eunicida, Annelida) and the phylogenetic utility of noncongruent 16S, COI and 18S in combined analyses. Molecular Phylogenetics and Evolution, 55(2), 660–676.
Acknowledgments
This work has been carried out during a research visit of ET to the Scripps Institution of Oceanography, which was funded by the German Academic Exchange Service (DAAD) (Grant No. 91536193-57044987). Our thanks are also due to the staff of the Laboratoire de Biologie Marine Concarneau (France, Brittany).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
Complete list of sequences used for the phylogenetic analysis with the corresponding GenBank sequence accession numbers. (DOCX 19 kb)
Rights and permissions
About this article
Cite this article
Tilic, E., Bartolomaeus, T. & Rouse, G.W. Chaetal type diversity increases during evolution of Eunicida (Annelida). Org Divers Evol 16, 105–119 (2016). https://doi.org/10.1007/s13127-015-0257-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-015-0257-z