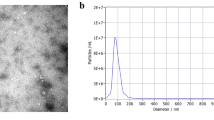
Abstract
MicroRNAs (miRNAs) play an important role in the pathogenesis of atrial fibrillation (AF). Exosomal miRNAs may develop as promising biomarkers for AF. To explore significant exosomal miRNAs in AF, plasma exosomes were extracted from 3 patients with AF and 3 patients with sinus rhythm (SR), respectively. Differential expression of exosomal miRNAs were screened by high-throughput sequencing analysis and verified by qRT-PCR from 40 patients with AF and 40 patients with SR. The target genes prediction, biological function, and signaling pathways analysis were conducted by miRanda software, gene ontology (GO), and KEGG analysis. The results showed that there were 40 differently expressed exosomal miRNAs from AF patients compared with SR patients, of which 13 miRNAs were upregulated and 27 miRNAs were downregulated. qRT-PCR validation demonstrated that miR-124-3p, miR-378d, miR-2110, and miR-3180-3p were remarkably upregulated, while miR-223-5p, miR-574-3p, miR-125a-3p, and miR-1299 were downregulated. To explore the function of miR-124-3p associated with AF, plasma exosomes derived from AF patients were co-incubated with rat myocardial fibroblasts. The expression of miR-124-3p was upregulated in myocardial fibroblasts. The viability and proliferation of myocardial fibroblasts were elevated by transfecting with miR-124-3p overexpression plasmids using CCK8 and immunofluorescence-staining methods. AXIN1 was verified to be the target of miR-124-3p by luciferase assay in vitro. Expression of AXIN1 was reduced, while β-catenin, Collagen 1, and α-SMA were increased in myocardial fibroblasts with miR-124-3p overexpression. In conclusion, these findings suggested that circulating exosomal miRNAs may serve as novel biomarkers for AF, and miR-124-3p promotes fibroblast activation and proliferation through regulating WNT/β-catenin signaling pathway via AXIN1.








Similar content being viewed by others
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Briasoulis A, Sharma S, Telila T, Mallikethi-Reddy S, Papageorgiou N, Oikonomou E, Tousoulis D (2019) MicroRNAs in atrial fibrillation. Curr Med Chem 26(5):855–863. https://doi.org/10.2174/0929867324666170920151024
Burstein B, Nattel S (2008) Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol 51(8):802–809. https://doi.org/10.1016/j.jacc.2007.09.064
Cardin S, Guasch E, Luo X, Naud P, Le Quang K, Shi Y, Tardif JC, Comtois P, Nattel S (2012) Role for MicroRNA-21 in atrial profibrillatory fibrotic remodeling associated with experimental postinfarction heart failure. Circ Arrhythm Electrophysiol 5(5):1027–1035. https://doi.org/10.1161/CIRCEP.112.973214
Clayton RH (2018) Dispersion of recovery and vulnerability to re-entry in a model of human atrial tissue with simulated diffuse and focal patterns of fibrosis. Front Physiol 9:1052. https://doi.org/10.3389/fphys.2018.01052
Mun D, Kim H, Kang JY, Park H, Park H, Lee SH, Yun N, Joung B (2019) Expression of miRNAs in circulating exosomes derived from patients with persistent atrial fibrillation. FASEB J 33(5):5979–5989. https://doi.org/10.1096/fj.201801758R
Dzeshka MS, Lip GY, Snezhitskiy V, Shantsila E (2015) Cardiac fibrosis in patients with atrial fibrillation: mechanisms and clinical implications. J Am Coll Cardiol 66(8):943–959. https://doi.org/10.1016/j.jacc.2015.06.1313
Enright AJ, John B, Gaul U, Tuschl T, Sander C, Marks DS (2003) MicroRNA targets in Drosophila. Genome Biol 5(1):R1. https://doi.org/10.1186/gb-2003-5-1-r1
E S, Costa MC, Kurc S, Drożdż A, Cortez-Dias N, Enguita FJ, (2018) The circulating non-coding RNA landscape for biomarker research: lessons and prospects from cardiovascular diseases. Acta Pharmacol Sin 39(7):1085–1099. https://doi.org/10.1038/aps.2018.35
Esteller M (2011) Non-coding RNAs in human disease. Nat Rev Genet 12(12):861–874. https://doi.org/10.1038/nrg3074
Frustaci A, Caldarulo M, Buffon A, Bellocci F, Fenici R, Melina D (1991) Cardiac biopsy in patients with “primary” atrial fibrillation. Histologic evidence of occult myocardial diseases. Chest 100(2):303–6. https://doi.org/10.1378/chest.100.2.303
Golden HB, Gollapudi D, Gerilechaogetu F, Li J, Cristales RJ, Peng X, Dostal DE (2012) Isolation of cardiac myocytes and fibroblasts from neonatal rat pups. Methods Mol Biol 843:205–214. https://doi.org/10.1007/978-1-61779-523-7_20
Griffiths-Jones S, Bateman A, Marshall M, Khanna A, Eddy SR (2003) Rfam: an RNA family database. Nucleic Acids Res 31(1):439–441. https://doi.org/10.1093/nar/gkg006
Guo Y, Xiao L, Sun L, Liu F (2012) Wnt/beta-catenin signaling: a promising new target for fibrosis diseases. Physiol Res 61(4):337–46. https://doi.org/10.33549/physiolres.932289
Hu G, Ma L, Dong F, Hu X, Liu S, Sun H (2019) Inhibition of microRNA-124-3p protects against acute myocardial infarction by suppressing the apoptosis of cardiomyocytes. Mol Med Rep 20(4):3379–3387. https://doi.org/10.3892/mmr.2019.10565
Iaconetti C, Sorrentino S, De Rosa S, Indolfi C (2016) Exosomal miRNAs in heart disease Physiology (Bethesda) 31(1):16–24. https://doi.org/10.1152/physiol.00029.2015
Knudsen L, Ruppert C, Ochs M (2017) Tissue remodelling in pulmonary fibrosis. Cell Tissue Res 367(3):607–626. https://doi.org/10.1007/s00441-016-2543-2
Köberle V, Pleli T, Schmithals C, Augusto Alonso E, Haupenthal J, Bönig H, Peveling-Oberhag J, Biondi RM, Zeuzem S, Kronenberger B, Waidmann O, Piiper A (2013) Differential stability of cell-free circulating microRNAs: implications for their utilization as biomarkers. PLoS ONE 8(9):e75184. https://doi.org/10.1371/journal.pone.0075184
Lin J, Li J, Huang B, Liu J, Chen X, Chen XM, Xu YM, Huang LF, Wang XZ (2015) Exosomes: novel biomarkers for clinical diagnosis. Sci World J 2015:657086. https://doi.org/10.1155/2015/657086
Liu B, Li J, Cairns MJ (2014) Identifying miRNAs, targets and functions. Brief Bioinform 15(1):1–19. https://doi.org/10.1093/bib/bbs075
Nattel S, Harada M (2014) Atrial remodeling and atrial fibrillation. J Am Coll Cardiol 63(22):2335–2345. https://doi.org/10.1016/j.jacc.2014.02.555
Platonov PG, Mitrofanova LB, Orshanskaya V, Ho SY (2011) Structural abnormalities in atrial walls are associated with presence and persistency of atrial fibrillation but not with age. J Am Coll Cardiol 58(21):2225–2232. https://doi.org/10.1016/j.jacc.2011.05.061
Shan H, Zhang Y, Lu Y, Zhang Y, Pan Z, Cai B, Wang N, Li X, Feng T, Hong Y, Yang B (2009) Downregulation of miR-133 and miR-590 contributes to nicotine-induced atrial remodelling in canines. Cardiovasc Res 83(3):465–472. https://doi.org/10.1093/cvr/cvp130
Shen H, Wang J, Min J, Xi W, Gao Y, Yin L, Yu Y, Liu K, Xiao J, Zhang YF, Wang ZN (2018) Activation of TGF-beta1/alpha-SMA/Col I Profibrotic Pathway in fibroblasts by galectin-3 contributes to atrial fibrosis in experimental models and patients. Physiol Biochem 47(2):851–863. https://doi.org/10.1159/000490077 (Epub 2018 May 22)
Staerk L, Sherer JA, Ko D, Benjamin EJ, Helm RH (2017) Atrial fibrillation epidemiology, pathophysiology, and clinical outcomes. Circ Res 120(9):1501–1517. https://doi.org/10.1161/CIRCRESAHA.117.309732
Nattel S, Harada M (2014) Atrial remodeling and atrial fibrillation: recent advances and translational perspectives. J Am Coll Cardiol 63(22):2335–2345. https://doi.org/10.1016/j.jacc.2014.02.555
Tao H, Yang JJ, Shi KH, Li J (2016) Wnt signaling pathway in cardiac fibrosis: new insights and directions. Metabolism 65(2):30–40. https://doi.org/10.1016/j.metabol.2015.10.013
Théry C, Amigorena S, Raposo G, Clayton A (2006) Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. Chapter 3:Unit 3.22. https://doi.org/10.1002/0471143030.cb0322s30
Thery C, Zitvogel L, Amigorena S (2002) Exosomes: composition, biogenesis and function. Nat Rev Immunol 2(8):569–579. https://doi.org/10.1038/nri855
Vaze A, Tran KV, Tanriverdi K, Sardana M, Lessard D, Donahue JK, Barton B, Aurigemma G, Lubitz SA, Lin H, Nasr GH, Mandapati A, Benjamin EJ, Vasan RS, Freedman JE (2020) McManus DD (2020) Relations between plasma microRNAs, echocardiographic markers of atrial remodeling, and atrial fibrillation: data from the Framingham Offspring study. PLoS ONE 15(8):e0236960. https://doi.org/10.1371/journal.pone.0236960.eCollection
Wang S, Min J, Yu Y, Yin L, Wang Q, Shen H, Yang J, Zhang P, Xiao J, Wang Z (2019) Differentially expressed miRNAs in circulating exosomes between atrial fibrillation and sinus rhythm. J Thorac Dis 11(10):4337–4348. https://doi.org/10.21037/jtd.2019.09.50
Yang W, Cui G, Ding M, Yang M, Dai D (2020) MicroRNA-124–3p.1 promotes cell proliferation through Axin1-dependent Wnt signaling pathway and predicts a poor prognosis of triple-negative breast cancer. J Clin Lab Anal 34(7):e23266. https://doi.org/10.1002/jcla.23266
Yue L, Xie J, Nattel S (2011) Molecular determinants of cardiac fibroblast electrical function and therapeutic implications for atrial fibrillation. Cardiovasc Res 89(4):744–753. https://doi.org/10.1093/cvr/cvq329
Zimetbaum P (2017) Atrial fibrillation. Ann Intern Med 166(5):ITC33–ITC48. https://doi.org/10.7326/AITC201703070
Zhang L, Dong R, Wei S, Zhou HC, Zhang MX, Alagarsamy K (2019) A novel data processing method CyC* for quantitative real time polymerase chain reaction minimizes cumulative error. PLoS ONE 14(6):e0218159. https://doi.org/10.1371/journal.pone.0218159
Zhang Y, Zheng S, Geng Y, Xue J, Wang Z, Xie X, Wang J, Zhang S, Hou Y (2015) MicroRNA profiling of atrial fibrillation in canines: MiR-206 modulates intrinsic cardiac autonomic nerve remodeling by regulating SOD1. PLoS ONE 10(3):e0122674. https://doi.org/10.1371/journal.pone.0122674
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers 81770334, 81970281); Taishan Scholar Engineering Construction Fund of Shandong Province (grant number ts201511104); Academic promotion programme of Shandong First Medical University (grant number 2019QL012); and Natural Science Foundation of Shandong Province (grant number ZR2020QH014).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Experimental funding acquisition, study guidance, and supervision were performed by Yujiao Zhang and Yinglong Hou. Material preparation, data collection, and analysis were performed by Huilin Li, An Zhang, Zhan Li, Yong Zhang, and Manyi Ren. The first draft of the manuscript was written by Pengju Zhu. All authors commented on previous versions of the manuscript and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
This study was approved by the Ethics Committee of Shandong University (Approval No. 2018S0021).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Disclaimer
The authors declare that all data were generated in-house and that no paper mill was used.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
There was differential expression of exosomal miRNAs between the patients with AF and SR, in which miR-124-3p was significantly overexpressed in AF.
MiR-124-3p promotes rat cardiac fibroblast activation and proliferation by regulating WNT/β-catenin pathway via AXIN1.
Rights and permissions
About this article
Cite this article
Zhu, P., Li, H., Zhang, A. et al. MicroRNAs sequencing of plasma exosomes derived from patients with atrial fibrillation: miR-124-3p promotes cardiac fibroblast activation and proliferation by regulating AXIN1. J Physiol Biochem 78, 85–98 (2022). https://doi.org/10.1007/s13105-021-00842-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-021-00842-9