Abstract
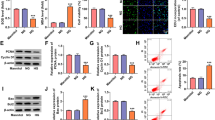
Epithelial-to-mesenchymal transition (EMT) and injury of tubular cells play critical roles in the pathogenesis of diabetic nephropathy (DN). lncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) has been shown to be involved in DN progression. However, whether MALAT1 induces EMT and injury in tubular cells is unclear. Here, we investigated the effects of MALAT1 on human proximal tubular cells (HK-2 cells) and the underlying mechanism. We performed qPCR to detect MALAT1, E-cadherin, α-smooth muscle actin (α-SMA), kidney injury molecule 1 (KIM-1), and neutrophil gelatinase-associated lipocalin (NGAL). Additionally, we conducted Western blot analyses to measure E-cadherin, α-SMA, cyclin D1, c-Myc, and β-catenin in HK-2 cells cultured with normal glucose and high glucose (HG) and in transfected cells or cells treated with LiCl and DKK-1. The β-catenin localization was observed using immunofluorescence, and the protein levels of NGAL and KIM-1 were evaluated by ELISA. We found that HG-upregulated MALAT1 decreased E-cadherin and increased α-SMA, KIM-1, NGAL, cyclin D1, c-Myc, and β-catenin in HK-2 cells. LiCl exposure increased the expression of α-SMA but decreased that of E-cadherin on the base of knocking down MALAT1, and decreased NGAL and KIM-1 expression. DKK-1 showed the opposite effects. Our results suggested that upregulated MALAT1 induced EMT in HG-treated HK-2 cells through activating the Wnt/β-catenin pathway. However, MALAT1-mediated injury in HK-2 cells was not mediated by activation of the Wnt/β-catenin pathway. Our results indicate that MALAT1 might serve as a novel therapeutic target for suppressing the progression of DN.






Similar content being viewed by others
References
Alvarez ML, Khosroheidari M, Eddy E, Kiefer J (2013) Role of microRNA 1207-5P and its host gene, the long non-coding RNA Pvt1, as mediators of extracellular matrix accumulation in the kidney: implications for diabetic nephropathy. PLoS One 8:e77468. https://doi.org/10.1371/journal.pone.0077468
Beltrami C, Angelini TG, Emanueli C (2015) Noncoding RNAs in diabetes vascular complications. J Mol Cell Cardiol 89:42–50. https://doi.org/10.1016/j.yjmcc.2014.12.014
Chen S, Dong C, Qian X, Huang S, Feng Y, Ye X, Miao H, You Q, Lu Y, Ding D (2017) Microarray analysis of long noncoding RNA expression patterns in diabetic nephropathy. J Diabetes Complicat 31:569–576. https://doi.org/10.1016/j.jdiacomp.2016.11.017
Dai C, Stolz D, Kiss L, Monga S, Holzman L, Liu Y (2009) Wnt/beta-catenin signaling promotes podocyte dysfunction and albuminuria. J Am Soc Nephrol 20:1997–2008
Grande MT, Sanchez-Laorden B, Lopez-Blau C, De Frutos CA, Boutet A, Arevalo M, Rowe RG, Weiss SJ, Lopez-Novoa JM, Nieto MA (2015) Snail1-induced partial epithelial-to-mesenchymal transition drives renal fibrosis in mice and can be targeted to reverse established disease. Nat Med 21:989–997. https://doi.org/10.1038/nm.3901
Guo Y, Li Z, Ding R, Li H, Zhang L, Yuan W, Wang Y (2014) Parathyroid hormone induces epithelial-to-mesenchymal transition via the Wnt/beta-catenin signaling pathway in human renal proximal tubular cells. Int J Clin Exp Pathol 7:5978–5987
Hu C, Sun L, Xiao L, Han Y, Fu X, Xiong X, Xu X, Liu Y, Yang S, Liu F, Kanwar YS (2015) Insights into the mechanisms involved in the expression and regulation of extracellular matrix proteins in diabetic nephropathy. Curr Med Chem 22:2858–2870
Huang BY, Cao LY, Fu XG (2012) Effects of tanshinone IIA on Wnt/beta-catenin signaling pathway of high glucose induced renal tubular epithelial cell transdifferentiation. Zhongguo Zhong Xi Yi Jie He Za Zhi 32:965–969
Ina K, Kitamura H, Tatsukawa S, Miyazaki T, Abe H, Fujikura Y (2007) Contraction of tubulointerstitial fibrosis tissue in diabetic nephropathy, as demonstrated in an in vitro fibrosis model. Virchows Arch 451:911–921. https://doi.org/10.1007/s00428-007-0511-7
Inoue T, Umezawa A, Takenaka T, Suzuki H, Okada H (2015) The contribution of epithelial-mesenchymal transition to renal fibrosis differs among kidney disease models. Kidney Int 87:233–238. https://doi.org/10.1038/ki.2014.235
Ji P, Diederichs S, Wang W, Boing S, Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E, Thomas M, Berdel WE, Serve H, Muller-Tidow C (2003) MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene 22:8031–8041. https://doi.org/10.1038/sj.onc.1206928
Ji Q, Liu X, Fu X, Zhang L, Sui H, Zhou L, Sun J, Cai J, Qin J, Ren J, Li Q (2013) Resveratrol inhibits invasion and metastasis of colorectal cancer cells via MALAT1 mediated Wnt/beta-catenin signal pathway. PLoS One 8:e78700. https://doi.org/10.1371/journal.pone.0078700
Kawakami T, Ren S, Duffield JS (2013) Wnt signalling in kidney diseases: dual roles in renal injury and repair. J Pathol 229:221–231. https://doi.org/10.1002/path.4121
LeBleu V, Taduri G, O'Connell J, Teng Y, Cooke V, Woda C, Sugimoto H, Kalluri R (2013) Origin and function of myofibroblasts in kidney fibrosis. Nat Med 19:1047–1053
Lee YJ, Han HJ (2010) Troglitazone ameliorates high glucose-induced EMT and dysfunction of SGLTs through PI3K/Akt, GSK-3beta, Snail1, and beta-catenin in renal proximal tubule cells. Am J Physiol Ren Physiol 298:F1263–F1275. https://doi.org/10.1152/ajprenal.00475.2009
Li Y, Sun Y, Liu F, Sun L, Li J, Duan S, Liu H, Peng Y, Xiao L, Liu Y, Xi Y, You Y, Li H, Wang M, Wang S, Hou T (2013) Norcantharidin inhibits renal interstitial fibrosis by blocking the tubular epithelial-mesenchymal transition. PLoS One 8:e66356. https://doi.org/10.1371/journal.pone.0066356
Li X, Zeng L, Cao C, Lu C, Lian W, Han J, Zhang X, Zhang J, Tang T, Li M (2017) Long noncoding RNA MALAT1 regulates renal tubular epithelial pyroptosis by modulated miR-23c targeting of ELAVL1 in diabetic nephropathy. Exp Cell Res 350:327–335. https://doi.org/10.1016/j.yexcr.2016.12.006
Liang J, Liang L, Ouyang K, Li Z, Yi X (2017) MALAT1 induces tongue cancer cells’ EMT and inhibits apoptosis through Wnt/beta-catenin signaling pathway. J Oral Pathol Med 46:98–105. https://doi.org/10.1111/jop.12466
Louis K, Hertig A (2015) How tubular epithelial cells dictate the rate of renal fibrogenesis? World J Nephrol 4:367–373. https://doi.org/10.5527/wjn.v4.i3.367
Lovisa S, LeBleu VS, Tampe B, Sugimoto H, Vadnagara K, Carstens JL (2015) Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat Med 21:998–1009. https://doi.org/10.1038/nm.3902
Lovisa S, Zeisberg M, Kalluri R (2016) Partial epithelial-to-mesenchymal transition and other new mechanisms of kidney fibrosis. Trends Endocrinol Metab 27:681–695. https://doi.org/10.1016/j.tem.2016.06.004
Menon MC, Ross MJ (2016) Epithelial-to-mesenchymal transition of tubular epithelial cells in renal fibrosis: a new twist on an old tale. Kidney Int 89:263–266. https://doi.org/10.1016/j.kint.2015.12.025
Nelson PJ, von Toerne C, Grone HJ (2011) Wnt-signaling pathways in progressive renal fibrosis. Expert Opin Ther Targets 15:1073–1083. https://doi.org/10.1517/14728222.2011.588210
Puthanveetil P, Chen S, Feng B, Gautam A, Chakrabarti S (2015) Long non-coding RNA MALAT1 regulates hyperglycaemia induced inflammatory process in the endothelial cells. J Cell Mol Med 19:1418–1425. https://doi.org/10.1111/jcmm.12576
Schmitz SU, Grote P, Herrmann BG (2016) Mechanisms of long noncoding RNA function in development and disease. Cellular and molecular life sciences. CMLS 73:2491–2509. https://doi.org/10.1007/s00018-016-2174-5
Tan RJ, Zhou D, Liu Y (2016) Signaling crosstalk between tubular epithelial cells and interstitial fibroblasts after kidney injury. Kidney Dis (Basel, Switzerland) 2:136–144. https://doi.org/10.1159/000446336
Wang JY, Yang JH, Xu J, Jia JY, Zhang XR, Yue XD, Chen LM, Shan CY, Zheng MY, Han F, Zhang Y, Yang XY, Chang BC (2015) Renal tubular damage may contribute more to acute hyperglycemia induced kidney injury in non-diabetic conscious rats. J Diabetes Complicat 29:621–628. https://doi.org/10.1016/j.jdiacomp.2015.04.014
Wong DW, Yiu WH, Wu HJ, Li RX, Liu Y, Chan KW, Leung JC, Chan LY, Lai KN, Tang SC (2016) Downregulation of renal tubular Wnt/beta-catenin signaling by Dickkopf-3 induces tubular cell death in proteinuric nephropathy. Cell Death Dis 7:e2155. https://doi.org/10.1038/cddis.2016.62
Yan B, Tao ZF, Li XM, Zhang H, Yao J, Jiang Q (2014) Aberrant expression of long noncoding RNAs in early diabetic retinopathy. Invest Ophthalmol Vis Sci 55:941–951. https://doi.org/10.1167/iovs.13-13221
Zhao Y, Yin Z, Li H, Fan J, Yang S, Chen C, Wang DW (2017) MiR-30c protects diabetic nephropathy by suppressing epithelial-to-mesenchymal transition in db/db mice. Aging Cell 16:387–400. https://doi.org/10.1111/acel.12563
Zhou L, Liu Y (2015) Wnt/beta-catenin signalling and podocyte dysfunction in proteinuric kidney disease. Nat Rev Nephrol 11:535–545. https://doi.org/10.1038/nrneph.2015.88
Zhou L, Li Y, Hao S, Zhou D, Tan R, Nie J, Hou F, Kahn M, Liu Y (2015) Multiple genes of the renin-angiotensin system are novel targets of Wnt/β-catenin signaling. J Am Soc Nephrol 26:107–120
Zhou L, Xu DY, Sha WG, Shen L, Lu GY, Yin X, Wang MJ (2015) High glucose induces renal tubular epithelial injury via Sirt1/NF-kappaB/microR-29/Keap1 signal pathway. J Transl Med 13:352. https://doi.org/10.1186/s12967-015-0710-y
Funding
This work was supported by the National Natural Science Foundation of China (No. 81202842).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical statement
Ethics Committee approval was obtained from the Institutional Ethics Committee of Zhujiang Hospital of Southern Medical University to the commencement of the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, J., Jiang, T., Liang, X. et al. lncRNA MALAT1 mediated high glucose–induced HK-2 cell epithelial-to-mesenchymal transition and injury. J Physiol Biochem 75, 443–452 (2019). https://doi.org/10.1007/s13105-019-00688-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-019-00688-2