Abstract
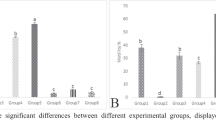
Recent studies have shown that lead (Pb) could disrupt tissue prooxidant/antioxidant balance which lead to physiological dysfunction. Natural antioxidants are particularly useful in such situation. Current study was designed to investigate efficacy of green tea extract (GTE), on oxidative status in brain tissue and blood caused by chronic oral Pb administration in rats. Four groups of adult male rats (each 15 rats) were utilized: control group; GTE-group (oral 1.5% w/v GTE for 6 weeks); Pb-group (oral 0.4% lead acetate for 6 weeks), and Pb+GTE-group (1.5% GTE and 0.4% lead acetate for 6 weeks). Levels of prooxidant/antioxidant parameters [lipid peroxides (LPO), nitric oxides (NO), total antioxidant capacity (TAC), glutathione (GSH), glutathione-S-transferase (GST), superoxide dismutase (SOD)] in plasma, erythrocytes, and brain tissue homogenate were measured using colorimetric methods. Pb concentrations in whole blood and brain tissue homogenate were measured by atomic absorption. In Pb-group, levels of LPO were higher while NO and GSH were lower in plasma, erythrocytes, and brain tissue than controls. TAC in plasma, SOD in erythrocytes, and GST in brain tissue homogenate were lower in Pb-group versus control. GTE co-administrated with Pb-reduced Pb contents, increased antioxidant status than Pb-group. In erythrocytes, Pb correlated positively with LPO and negatively with NO, GSH, SOD, and Hb. In brain tissue homogenate, Pb correlated positively with LPO and negatively with GSH. This study suggests that lead induce toxicity by interfering balance between prooxidant/antioxidant. Treatment of rats with GTE combined with Pb enhances antioxidant/ detoxification system which reduced oxidative stress. These observations suggest that GTE is a potential complementary agent in treatment of chronic lead intoxication.
Similar content being viewed by others
References
Adonaylo VN, Oteiza PI (1999) Lead intoxication: antioxidant defenses and oxidative damage in rat brain. Toxicology 135:77–85
Adonaylo VN, Oteiza PI (1999) Pb2+ promotes lipid oxidation and alterations in membrane physical properties. Toxicology 132:19–32
Bennet C, Rajanna B, Sharada R, Baker L, Yallapragada PR, Brice JJ, White SL, Kumar BK (2007) Region specific increase in the antioxidant enzymes and lipid peroxidation products in the brain of rats exposed to lead. Free Radic Res 41:267–273
Berrahal A, Nehdi A, Hajjaji N, Gharbi N, El-Fazâ S (2007) Antioxidant enzymes activities and bilirubin level in adult rat treated with tea. C R Biol 330(8):581–588
Bokara KK, Blaylock I, Denise SB, Bettaiya R, Rajanna S, Yallapragada PR (2009) Influence of lead acetate on glutathione and its related enzymes in different regions of rat brain. J Appl Toxicol 29(5):452–458
Bressler J, Kim KA, Chakraborti T, Goldstein G (1999) Mechanism of lead neurotoxicity. Neurochem Res 24:595–600
Campbell EL, Chebib M, Johnston GAR (2004) The dietary flavonoids apigenin and (−)-epigallocatechin gallate enhance the positive modulation by diazepam of the activation by GABA of recombinant GABAA receptors. Biochem Pharmacol 68:1631–1638
Carmignani M, Volp A, Paolo D, Qiao N, Gioacchino MD (2000) Catcholamine and nitric oxide systems as targets of chronic lead exposure in inducing selective functional impairment. Life Sci 68:401–415
Chan MM, Fong D, Ho CT, Huang HI (1997) Inhibition of inducible nitric oxide synthase gene expression and enzyme activity by epigallocatechin gallate, a natural product from green tea. Biochem Pharmacol 54:1281–1286
Christian GD (1969) Medicine, trace metals and atomic absorption spectroscopy. Ann Chem 41:24A–40A
Costa CA, Trivelato GC, Pinto AM, Bechara EJ (1997) Correlation between plasma 5-aminolevulinic acid concentrations and indicators of oxidative stress in lead-exposed workers. Clin Chem 43:1196–1202
Daniel S, Limson JL, Amichand D, Watkins GM, Daya S (2004) Through metal binding, curcumin protects against lead- and cadmium-induced lipid peroxidation in rat brain homogenates and against lead-induced tissue damage in rat brain. J Inorg Biochem 98:266–275
Ding Y, Gonick HC, Vaziri ND (2000) Lead promotes hydroxyl radical generation and lipid peroxidation in cultured aortic endothelial cells. Am J Hypertens 13:552–555
Dutta P, Seirafi J, Halpin D, Pinto J, Rivlin R (1995) Acute ethanol exposure alters hepatic glutathione metabolism in riboflavin deficiency. Alcohol 12:43–47
Erba D, Riso P, Bordoni A, Foti P, Biagi PL, Testolin G (2005) Effectiveness of moderate green tea consumption on antioxidative status and plasma lipid profile in humans. J Nutr Biochem 16:144–149
Flora SJS, Mittal M, Mehta A (2008) Heavy metal induced oxidative stress and its possible reversal by chelation therapy. Indian J Med Res 128:501–523
Guilarte TR, McGlothan JL (2003) Selective decrease in NR1 subunit splice variant mRNA in the hippocampus of Pb2+-exposed rats: implications for synaptic targeting and cell surface expression of NMDAR complexes. Brain Res Mol Brain Res 113:37–43
Guo Q, Zhao B, Li M, Shen S, Xin W (1996) Studies on protective mechanisms of four components of green tea polyphenols against lipid peroxidation in synaptosomes. Biochim Biophys Acta 1304:210–222
Gurer H, Ozgunes H, Neal R, Spitzand DR, Ercal N (1998) Antioxidant effects of N-acetyl cystein and succimer in red blood cells from lead exposed rats. Toxicology 128:181–189
Gurer H, Neal R, Yang P, Oztezcan S, Erçal N (1999) Captopril as an antioxidant in lead-exposed Fischer 344 rats. Hum Exp Toxicol 18:27–32
Habig WH, Pabst MJ, Jakoby WB (1973) Glutathione-S-transferases: the first enzymatic step in mercaturic acid formation. J Biol Chem 249:7130–7139
Hunaiti AA, Soud M (2000) Effect of lead concentration on the level of glutathione, glutathione S-transferase, reductase and peroxidase in human blood. Sci Total Environ 248:45–50
Ito Y, Niiya Y, Kurita H, Shima S, Sarai S (1985) Serum lipid peroxide level and blood superoxide dismutase activity in workers with occupational exposure to lead. Int Arch Occup Environ Health 56:119–127
Jacob B, Ritz B, Heinrich J, Hoelscher B, Wichmann HE (2000) The effect of low-level blood lead on hematologic parameters in children. Environ Res 82:150–159
Lee SR, Im KJ, Suh SI, Jung JG (2003) Protective effect of green tea polyphenol (−)-epigallocatechin gallate and other antioxidants on lipid peroxidation in gerbil brain homogenates. Phytother Res 17(3):206–209
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Maity S, Vadasiromoni J, Ganguly D (1998) Role of glutathione in the antiulcer effect of hot water extract of black tea. Jpn J Pharmacol 78:285–292
Mandel S, Weinreb O, Reznichenk L, Kafon L, Amit T (2006) Green tea catechins as brain-permeable, non toxic iron chelators to 'iron out iron' from the brain. J Neural Transm 71:249–257
Misra HP, Fridovich I (1972) The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247(10):3170–3175
Mohammad IK, Mahdi AA, Raviraja A, Najmul I, Iqbal A, Thuppil V (2008) Oxidative stress in painters exposed to low lead levels. Arh Hig Rada Toksikol 59(3):161–169
Pagila DE, Valentine WN, Dahlger JG (1976) Effects of low level lead exposure on pyrimidine-5' nucleotidase and other erythrocytes enzymes. J Clin Invest 56:1164–1169
Patil AJ, Bhagwat VR, Patil JA, Dongre NN, Ambekar JG, Jailkhani R, Das KK (2006) Effect of lead (Pb) exposure on the activity of superoxide dismutase and catalase in battery manufacturing workers (BMW) of western Maharashtra (India) with reference to heme biosynthesis. Int J Environ Res Public Health 3:329–337
Patra RC, Swarup D, Dwivedi S (2001) Antioxidant effects of α-tocopherol, ascorbic acid and l-methionine on lead-induced oxidative stress to the liver, kidney and brain in rats. Toxicology 162:81–88
Rao GM, Shetty BV, Sudha K (2007) Evaluation of lead toxicity and antioxidants in battery workers. Biomedical Research 19(1):1–4
Reiter RL (1995) Oxidative processes and antioxidative defense mechanisms in the aging brain. FASEBJ 9:526–533
Saxena G, Flora SJS (2004) Lead induced oxidative stress and hematological alterations and their response to combined administration of calcium disodium EDTA with a thiol chelator in rats. J Biochem & Molecular Toxicology 18:221–233
Shalan MG, Mostafa MS, Hassouna MM, El-Nabi HSE, El-Rafaie A (2005) Amelioration of lead toxicity on rat liver with vitamin C and silymarin supplements. Toxicology 206:1–15
Shin BC, Ryu HH, Chung JH, Kim HL (2009) The protective effects of green tea extract against l-arginine toxicity to cultured human mesangial cells. J Korean Med Sci 24(1):S204–S209
Singh R, Ahmed S, Islam N, Goldberg VM, Haqqi TM (2002) Epigallocatechin-3-gallate inhibits interleukin-1beta-induced expression of nitric oxide synthase and production of nitric oxide in human chondrocytes: suppression of nuclear factor kappaB activation by degradation of the inhibitor of nuclear factor kappaB. Arthritis Rheum 46(8):2079–2086
Sivaprasad R, Nagaraj M, Varalakshmi P (2003) Combined efficacies of lipoic acid and meso-2,3-dimercaptosuccinic acid on lead induced erythrocyte membrane lipid peroxidation and antioxidant status in rats. Hum Exp Toxicol 22:183–192
Sivaprasad RT, Malarkodi SP, Varalakshmi P (2004) Therapeutic efficacy of lipoic acid combination with dimercaptosuccinic acid against lead-induced renal tubular defects and tubular defects and on isolated bruch-border enzyme activities. Chem Biol Interact 147(3):259–271
Skrzydlewska E, Ostrowska J, Farbiszewski R, Michalak K (2002) Protective effect of green tea against lipid peroxidation in the rat liver, blood serum and the brain. Phytomedicine 9:232–238
Stohs SJ, Bagchi D (1995) Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med 18:321–36
Sugawara E, Nakamura K, Miyake T, Fukumura A, Seki Y (1991) lipid peroxidation and concentration of glutathione in erythrocytes from workers exposed to lead. Br J Ind Med 48:239–242
Thayer WS (1984) Serum lipid peroxides in rats treated chronically with adriamycin. Biochem Pharmacol 33(14):2259–2263
van Bezooijen RL, Que I, Ederveen AG, Kloosterboer HJ, Papapoulos SE, Lowik CW (1998) Plasma nitrate+nitrite level are regulated by ovarian steroids but do not correlate with trabecular bone mineral density in rats. J Endocrinol 159:27–34
Villeda-Hernandez J, Barroso-Moguel R, Mendez-Armenta M, Nava-Ruiz C, Huerta-Romero R, Rios C (2001) Enhanced brain regional lipid peroxidation in developing rats exposed to low level lead acetate. Brain Res Bull 55:247–251
Wasowicz W, Gromadizinska J, Rydzynski K (2001) Blood concentration of essential trace elements and heavy metals in workers exposed to lead and cadmium. Int J Occup Med Environ Health 14:223–229
Ye XB, Fu H, Zhu JL, Ni WM, Lu YW, Kuang XY, Yang SL, Shu BX (1999) A study on oxidative stress in lead-exposed workers. J Toxicol Environ Health A 57:161–172
Yin ST, Tang ML, Su L, Chen L, Hu P, Wang HL, Wang M, Ruan DY (2008) Effects of epigallocatechin-3-gallate on lead-induced oxidative damage. Toxicology 249(1):45–54
Youdim KA, Shukitt-Hale B, MacKinnon S, Kalt W, Joseph JA (2000) Polyphenols enhance red blood cell resistance to oxidative stress in vitro and in vivo. Biochim Biophys Acta 1523(1):117–122
Zhu ZW, Yang RL, Dong GL, Zhao ZY (2005) Study on the neurotoxic effects of low-level lead exposure in rats. J Zhejiang Univ Sci B 6(7):686–692
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hamed, E.A., Meki, AR.M.A. & Abd El-Mottaleb, N.A. Protective effect of green tea on lead-induced oxidative damage in rat’s blood and brain tissue homogenates. J Physiol Biochem 66, 143–151 (2010). https://doi.org/10.1007/s13105-010-0019-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-010-0019-5