Abstract
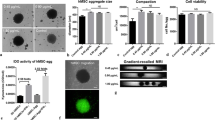
Compromised adult human mesenchymal stem cells (hMSC) can impair cell therapy efficacy and further reverse ischemic recovery. However, in vitro assays require extended passage to characterize cells, limiting rapid assessment for therapeutic potency. Multinuclear magnetic resonance imaging and spectroscopy (MRI/S) provides near real-time feedback on disease progression and tissue recovery. Applied to ischemic stroke, 23Na MRI evaluates treatment efficacy within 24 h after middle cerebral artery occlusion, showing recovery of sodium homeostasis and lesion reduction in specimens treated with hMSC while 1H MRS identifies reduction in lactate levels. This combined metric was confirmed by evaluating treatment groups receiving healthy or compromised hMSC versus vehicle (sham saline injection) over 21 days. Behavioral tests to assess functional recovery and cell analysis for immunomodulatory and macrophage activity to detect hMSC potency confirm MR findings. Clinically, these MR metrics may prove critical to early evaluations of therapeutic efficacy and overall stroke recovery.







Similar content being viewed by others
Data Availability
All data used in this study were newly acquired. Relevant data used in preparation of this manuscript are available upon request to either the principal or corresponding author via email. In accordance with the National High Magnetic Field Laboratory FAIR Data Management Plan (www.nationalmaglab.org/about/fair-data), data can be made available for access through the NHMFL publication database.
References
Trounson A, McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015;17:11–22.
Squillaro T, Peluso G, Galderisi U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant. 2016;25:829–48. https://doi.org/10.3727/096368915X689622.
Wang J, Liao L, Tan J. Mesenchymal-stem-cell-based experimental and clinical trials: current status and open questions. Expert Opin Biol Ther. 2011;11:893–909.
Zheng H, Zhang B, Chhatbar PY, Dong Y, Alawieh A, Lowe F, et al. Mesenchymal stem cell therapy in stroke: a systematic review of literature in pre-clinical and clinical research. Cell Transplant. 2018;27:1723–30.
Honmou O, Onodera R, Sasaki M, Waxman SG, Kocsis JD. Mesenchymal stem cells: therapeutic outlook for stroke. Trends Mol Med. 2012;18:292–7.
Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Ishii K, Kobune M, et al. Mesenchymal stem cells that produce neurotrophic factors reduce ischemic damage in the rat middle cerebral artery occlusion model. Mol Ther. 2005;11:96–104.
Yuan X, Rosenberg JT, Liu Y, Grant SC, Ma T. Aggregation of human mesenchymal stem cells enhances survival and efficacy in stroke treatment. Cytotherapy. 2019;21:1033–48. https://doi.org/10.1016/j.jcyt.2019.04.055.
Li Y, Dong Y, Ran Y, Zhang Y, Wu B, Xie J, et al. 2021 Three-dimensional cultured mesenchymal stem cells enhance repair of ischemic stroke through inhibition of microglia. Stem Cell Res Ther;12. https://doi.org/10.1186/S13287-021-02416-4
Widholz B, Tsitlakidis S, Reible B, Moghaddam A, Westhauser F. Pooling of patient-derived mesenchymal stromal cells reduces inter-individual confounder-associated variation without negative impact on cell viability, proliferation and osteogenic differentiation. Cells. 2019;8:633.
Kubo H, Takamura K, Nagaya N, Ohgushi H. The effect of serum on the proliferation of bone marrow-derived mesenchymal stem cells from aged donors and donors with or without chronic heart failure. J Tissue Eng Regen Med. 2018;12:e395–7. https://doi.org/10.1002/TERM.2394.
Bragdon B, Burns R, Baker AH, Belkina AC, Morgan EF, Denis GV, et al. Intrinsic sex-linked variations in osteogenic and adipogenic differentiation potential of bone marrow multipotent stromal cells. J Cell Physiol. 2015;230:296–307. https://doi.org/10.1002/JCP.24705.
Coipeau P, Rosset P, Langonn A, Gaillard J, Delorme B, Rico A, et al. Impaired differentiation potential of human trabecular bone mesenchymal stromal cells from elderly patients. Cytotherapy. 2009;11:584–94. https://doi.org/10.1080/14653240903079385.
Wahl EA, Schenck TL, Machens HG, Egaña JT. Acute stimulation of mesenchymal stem cells with cigarette smoke extract affects their migration, differentiation and paracrine potential. Sci Reports. 2016;61(6):1–9. https://doi.org/10.1038/srep22957.
Zaim M, Karaman S, Cetin G, Isik S. Donor age and long-term culture affect differentiation and proliferation of human bone marrow mesenchymal stem cells. Ann Hematol. 2012;91:1175–86. https://doi.org/10.1007/S00277-012-1438-X/FIGURES/7.
Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–82.
Ramos-Cabrer P, Hoehn M. MRI stem cell tracking for therapy in experimental cerebral ischemia. Transl Stroke Res. 2012;3:22–35.
Nguyen PK, Riegler J, Wu JC. Stem cell imaging: from bench to bedside. Cell Stem Cell. 2014;14:431–44.
Shapiro EM, Skrtic S, Sharer K, Hill JM, Dunbar CE, Koretsky AP. MRI detection of single particles for cellular imaging. Proc Natl Acad Sci U S A. 2004;101:10901–6.
Shapiro EM, Sharer K, Skrtic S, Koretsky AP. In vivo detection of single cells by MRI. Magn Reson Med. 2006;55:242–9. https://doi.org/10.1002/mrm.20718.
Birenbaum D, Bancroft LW, Felsberg GJ 2011. Imaging in acute stroke. West J Emerg Med XII 67–76.
Wetterling F, Chatzikonstantinou E, Tritschler L, Meairs S, Fatar M, Schad LR, et al. 2016 Investigating potentially salvageable penumbra tissue in an in vivo model of transient ischemic stroke using sodium, diffusion, and perfusion magnetic resonance imaging. BMC Neurosci.17.
Hu H-J, Song M. Disrupted ionic homeostasis in ischemic stroke and new therapeutic targets. J Stroke Cerebrovasc Dis. 2017;26:2706–19. https://doi.org/10.1016/j.jstrokecerebrovasdis.2017.09.011.
Wetterling F, Ansar S, Handwerker E. Sodium-23 magnetic resonance imaging during and after transient cerebral ischemia: multinuclear stroke protocols for double-tuned 23Na/ 1H resonator systems. Phys Med Biol. 2012;57:6929–46.
Shemesh N, Dumez J-N, Frydman L. Longitudinal relaxation enhancement in 1 H NMR spectroscopy of tissue metabolites via spectrally selective excitation. Chem A Eur J. 2013;19:13002–8. https://doi.org/10.1002/chem.201300955.
Shemesh N, Rosenberg JT, Dumez J-N, Muniz JA, Grant SC, Frydman L. 2014 Metabolic properties in stroked rats revealed by relaxation-enhanced magnetic resonance spectroscopy at ultrahigh fields. Nat Commun.5. https://doi.org/10.1038/ncomms5958
Rosenberg JT, Shemesh N, Muniz JA, Dumez J-N, Frydman L, Grant SC. Transverse relaxation of selectively excited metabolites in stroke at 21 1 T. Magn Reson Med. 2017;77:520–8. https://doi.org/10.1002/mrm.26132.
Yuan X, Tsai A-C, Farrance I, Rowley JA, Ma T. Aggregation of culture expanded human mesenchymal stem cells in microcarrier-based bioreactor. Biochem Eng J. 2018;131:39–46. https://doi.org/10.1016/j.bej.2017.12.011.
Sart S, Bejarano FC, Baird MA, Yan Y, Rosenberg JT, Ma T, et al. Intracellular labeling of mouse embryonic stem cell–derived neural progenitor aggregates with micron-sized particles of iron oxide. Cytotherapy. 2015;17:98–111.
Guo L, Ge J, Zhou Y, Wang S, Zhao RCH, Wu Y. Three-dimensional spheroid-cultured mesenchymal stem cells devoid of embolism attenuate brain stroke injury after intra-arterial injection. Stem Cells Dev. 2014;23:978–89. https://doi.org/10.1089/scd.2013.0338.
Tsai A-C, Liu Y, Yuan X, Ma T. Compaction, fusion, and functional activation of three-dimensional human mesenchymal stem cell aggregate. Tissue Eng Part A. 2015;21:1705–19. https://doi.org/10.1089/ten.TEA.2014.0314.
Tsai A-C, Liu Y, Yuan X, Chella R, Ma T. Aggregation kinetics of human mesenchymal stem cells under wave motion. Biotechnol J. 2017;12:1600448. https://doi.org/10.1002/biot.201600448.
Bejoy J, Song L, Zhou Y, Li Y. Wnt/Yes-associated protein interactions during neural tissue patterning of human induced pluripotent stem cells. Tissue Eng Part A. 2018;24:546–58. https://doi.org/10.1089/TEN.TEA.2017.0153.
Song L, Yuan X, Jones Z, Vied C, Miao Y, Marzano M, et al. Functionalization of brain region-specific spheroids with isogenic microglia-like cells. Sci Reports. 2019;91(9):1–18. https://doi.org/10.1038/s41598-019-47444-6.
Hua TT, Bejoy J, Song L, Wang Z, Zeng Z, Zhou Y, et al. Cerebellar differentiation from human stem cells through retinoid. Wnt, and sonic hedgehog pathways. 2021;27:881–93. https://doi.org/10.1089/TEN.TEA.2020.0135.
Sart S, Liu Y, Ma T, Li Y. Microenvironment regulation of pluripotent stem cell-derived neural progenitor aggregates by human mesenchymal stem cell secretome. Tissue Eng Part A. 2014;20:2666–79.
Zhang Y, Yu S, Tuazon J, Lee JY, Corey S, Kvederis L, et al. Neuroprotective effects of human bone marrow mesenchymal stem cells against cerebral ischemia are mediated in part by an anti-apoptotic mechanism. Neural Regen Res. 2019;14:597–604. https://doi.org/10.4103/1673-5374.247464.
Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. https://doi.org/10.1161/01.str.20.1.84.
Fu R, Brey WW, Shetty K, Gorõkov P, Saha S, Long JR, et al. Ultra-wide bore 900 MHz high-resolution NMR at the National High Magnetic Field Laboratory. J Magn Reson. 2005;177:1–8.
Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13:129–53.
Schaar KL, Brenneman MM, Savitz SI. Functional assessments in the rodent stroke model. Exp Transl Stroke Med. 2010;2:13. https://doi.org/10.1186/2040-7378-2-13.
Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–87. https://doi.org/10.1016/S0028-3908(00)00005-8.
Chang CF, Niu KC, Hoffer BJ, Wang Y, Borlongan CV. Hyperbaric oxygen therapy for treatment of postischemic stroke in adult rats. Exp Neurol. 2000;166:298–306.
Helsper S, Bagdasarian FA, Yuan X, Xu K, Lee J-Y, Rosenberg JT, et al. 2022 Extended ischemic recovery after implantation of human mesenchymal stem cell aggregates indicated by sodium MRI at 21.1 T. Transl Stroke Res.; https://doi.org/10.1007/s12975-021-00976-4
Chen A, Siow B, Blamire AM, Lako M, Clowry GJ. Transplantation of magnetically labeled mesenchymal stem cells in a model of perinatal brain injury. Stem Cell Res. 2010;5:255–66.
Robson MD, Tyler DJ, Neubauer S. Ultrashort TE chemical shift imaging (UTE-CSI). Magn Reson Med. 2005;53:267–74. https://doi.org/10.1002/mrm.20344.
Wetterling F, Gallagher L, Mullin J, Holmes WM, McCabe C, Macrae IM, et al. Sodium-23 magnetic resonance imaging has potential for improving penumbra detection but not for estimating stroke onset time. J Cereb Blood Flow Metab. 2015;35:103–10.
Helsper S, Bagdasarian FA, Yuan X, Xu K, Lee J-Y, Rosenberg JT, et al. 2022 Ischemic recovery after implantation of human mesenchymal stem cells indicated by sodium MRI at 21.1 T. Transl Stroke Res. https://doi.org/10.1007/s12975-021-00976-4.
von Kummer R, Dzialowski I. 2017 Imaging of cerebral ischemic edema and neuronal death [Internet].Neuroradiology. p. 545–53. https://doi.org/10.1007/s00234-017-1847-6
Simard JM, Kent TA, Chen M, Tarasov K V., Gerzanich V. 2007 Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications [Internet]. Lancet Neurol.. p. 258–68. https://doi.org/10.1016/S1474-4422(07)70055-8
Parsons MW, Li T, Barber PA, Yang Q, Darby DG, Desmond PM, et al. Combined 1H MR spectroscopy and diffusion-weighted MRI improves the prediction of stroke outcome. Neurology. 2000;55:498–505. https://doi.org/10.1212/wnl.55.4.498.
Nemoto EM, Frinak S. Brain tissue pH after global brain ischemia and barbiturate loading in rats. Stroke. 1981;12:77–82. https://doi.org/10.1161/01.STR.12.1.77.
Immke DC, McCleskey EW. Lactate enhances the acid-sensing NA+ channel on ischemia-sensing neurons. Nat Neurosci. 2001;4:869–70. https://doi.org/10.1038/nn0901-869.
Muñoz Maniega S, Cvoro V, Chappell FM, Armitage PA, Marshall I, Bastin ME, et al. Changes in NAA and lactate following ischemic stroke: a serial MR spectroscopic imaging study. Neurology. 2008;71:1993–9.
Lin AQ, Shou JX, Li XY, Ma L, Zhu XH. Metabolic changes in acute cerebral infarction: Findings from proton magnetic resonance spectroscopic imaging. Exp Ther Med. 2014;7:451–5. https://doi.org/10.3892/etm.2013.1418.
Sager TN, Hansen AJ, Laursen H. Correlation between N-acetylaspartate levels and histopathologic changes in cortical infarcts of mice after middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2000;20:780–8. https://doi.org/10.1097/00004647-200005000-00004.
Sager TN, Fink-Jensen A, Hansen AJ. Transient elevation of interstitial n-acetylaspartate in reversible global brain ischemia. J Neurochem. 1997;68:675–82.
Sager TN, Henning L, Fink-Jensen A, Topp S, Stensgaard A, Hedehus M, et al. N-acetylaspartate distribution in rat brain striatum during acute brain ischemia. J Cereb Blood Metab. 1999;19:164–72.
Institute of Medicine (US) Committee on nutrition trauma and the brain. creatine. In: Erdman J, Oria M, Pillsbury L, editors. Nutr Trauma Brain Inj Improv Acute Subacute Heal Outcomes Mil Pers. 2011.
Turner CE, Byblow WD, Gant N. Creatine supplementation enhances corticomotor excitability and cognitive performance during oxygen deprivation. J Neurosci. 2015;35:1773–80. https://doi.org/10.1523/JNEUROSCI.3113-14.2015.
Rumpel H, Khoo JBK, Chang HM, Lim WEH, Chen C, Wong MC, et al. Correlation of the apparent diffusion coefficient and the creatine level in early ischemic stroke: a comparison of different patterns by magnetic resonance. J Magn Reson Imaging. 2001;13:335–43.
Kaneko Y, Lee J, Tajiri N, Tuazon JP, Lippert T, Russo E, et al. Translating intracarotid artery transplantation of bone marrow-derived NCS-01 cells for ischemic stroke: behavioral and histological readouts and mechanistic insights into stem cell therapy. Stem Cells Transl Med. 2020;9:203–20. https://doi.org/10.1002/sctm.19-0229.
Duggal S, Brinchmann JE. Importance of serum source for the in vitro replicative senescence of human bone marrow derived mesenchymal stem cells. J Cell Physiol. 2011;226:2908–15.
Estrada JC, Torres Y, Benguría A, Dopazo A, Roche E, Carrera-Quintanar L, et al. Human mesenchymal stem cell-replicative senescence and oxidative stress are closely linked to aneuploidy. Cell Death Dis. 2013;4(6):e691–e691. https://doi.org/10.1038/cddis.2013.211.
Tsai C-C, Su P-F, Huang Y-F, Yew T-L, Hung S-C. Molecular Cell Oct4 and Nanog directly regulate Dnmt1 to maintain self-renewal and undifferentiated state in mesenchymal stem cells. Mol Cell. 2012;47:169–82. https://doi.org/10.1016/j.molcel.2012.06.020.
Liu Y, Yuan X, Muñoz N, Logan TM, Ma T. Commitment to aerobic glycolysis sustains immunosuppression of human mesenchymal stem cells. Stem Cells Transl Med. 2018;8:93–106. https://doi.org/10.1002/sctm.18-0070.
Guan JL, Simon AK, Prescott M, Menendez JA, Liu F, Wang F, et al. Autophagy in stem cells. Autophagy. 2013;9:830–49. https://doi.org/10.4161/AUTO.24132.
Yuan X, Rosenberg JT, Liu Y, Grant SC, Ma T. Aggregation preconditioning enhances survival and efficacy of human mesenchymal stem cells in stroke treatment. Cytotherapy. 2019;21:1033–48. https://doi.org/10.1016/j.jcyt.2019.04.055.
François M, Romieu-Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20:187–95.
Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43:3063–70. https://doi.org/10.1161/STROKEAHA.112.659656.
Song N, Scholtemeijer M, Shah K. Mesenchymal stem cell immunomodulation: mechanisms and therapeutic potential. Trends Pharmacol Sci. 2020;41:653–64. https://doi.org/10.1016/j.tips.2020.06.009.
Faghihi R, Zeinali-Rafsanjani B, Mosleh-Shirazi MA, Saeedi-Moghadam M, Lotfi M, Jalli R, et al. 2017 Magnetic resonance spectroscopy and its clinical applications: a review [Internet]. J. Med. Imaging Radiat. Sci.. p. 233–53. https://doi.org/10.1016/j.jmir.2017.06.004
Funding
This study was funded by National Institutes of Health and the National Institute for Neurological Disorders and Stroke (RO1-NS102395 and F31-NS115409). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. A portion of this work was performed at the National High Magnetic Field Laboratory, which is supported by National Science Foundation Cooperative Agreement No. DMR-1644779 and the State of Florida.
Author information
Authors and Affiliations
Contributions
S.H. and S.C.G. conceived, designed, and directed the project. S.H. drafted the manuscript, performed all the surgical procedures, and acquired and interpreted all MR data. X.Y. designed and performed all the cell culture and in vitro analysis. J.A. performed and compiled the blind animal behavioral assessments and S.H. interpreted the results. F.A.B. assisted in animal handling and MR acquisition as needed. C.V.B and YL assisted with the interpretation. S.C.G. supervised the project. All the authors contributed to and approved the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed. All animal procedures were completed in accordance with the Animal Care and Use Committee at the Florida State University. All studies were conducted in accordance with the United States Public Health Service's Policy on Humane Care and Use of Laboratory Animals as well as the US NIH Guide for the Care and Use of Laboratory Animals (NIH Publications, No. 8023, 1978 revision).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Online Resource 1
(DOCX 809 kb)
Rights and permissions
About this article
Cite this article
Helsper, S., Yuan, X., Bagdasarian, F.A. et al. Multinuclear MRI Reveals Early Efficacy of Stem Cell Therapy in Stroke. Transl. Stroke Res. 14, 545–561 (2023). https://doi.org/10.1007/s12975-022-01057-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-022-01057-w