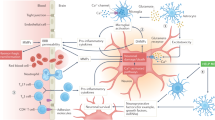
Abstract
Acute ischemic stroke is inadequately treated in the USA and worldwide due to a lengthy history of neuroprotective drug failures in clinical trials. The majority of victims must endure life-long disabilities that not only affect their livelihood, but also have an enormous societal economic impact. The rapid development of a neuroprotective or cytoprotective compound would allow future stroke victims to receive a treatment to reduce disabilities and further promote recovery of function. This opinion article reviews in detail the enormous costs associated with developing a small molecule to treat stroke, as well as providing a timely overview of the cell-death time-course and relationship to the ischemic cascade. Distinct temporal patterns of cell-death of neurovascular unit components provide opportunities to intervene and optimize new cytoprotective strategies. However, adequate research funding is mandatory to allow stroke researchers to develop and test their novel therapeutic approach to treat stroke victims.

Similar content being viewed by others
References
Poirier J, Derouesné D. VIII.9: Apoplexy and Stroke The Cambridge World History of Human Disease1993. p. 10.1017/CHOL9780521332866.071.
Wepfer J. Observationes anatomicae ex cadaveribus eorum quos sustulit apoplexia (cum exercitatione de eious loco affecto). Schaffussii: J C Suteri. 1658:670.
Gurdjian ES, Gurdjian ES. History of occlusive cerebrovascular disease I. From Wepfer to Moniz. Arch Neurol. 1979;36(6):340–3.
Willis T. Cerebri Anatome: cui accessit nervorum descriptio et usus. London; 1664.
Virchow R. Thrombose und Embolie: Gefässenzýndung und Septische Infektion, in Gesammelte Abhandlungen zur wissen schaftlichen Medicin: Frankfurt, a. M., Meidinger, Sohn und Co, 1856.
Dechambre A. Dictionnaire Encyclopédique des Sciences Médicales. Paris; 1866.
Bramwell B. Spontaneous meningeal haemorrhage. Edinburgh Medical Journal. 1886;32:101.
Symonds CP. Spontaneous sub-arachnoid haemorrhage. Proceedings of the Royal Society of Medicine. Neurol Sect. 1924;17:39–52.
Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association statistics committee and stroke statistics subcommittee. Circulation. 2009;119(3):480–6.
Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Executive summary: heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121(7):948–54.
Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46–e215.
Lapchak PA. Hemorrhagic transformation following ischemic stroke: significance, causes, and relationship to therapy and treatment. Curr Neurol Neurosci Rep. 2002;2(1):38–43.
Lyden PD, Zivin JA. Hemorrhagic transformation after cerebral ischemia: mechanisms and incidence. Cerebrovasc Brain Metab Rev. 1993;5(1):1–16.
Bernstein RA, Del-Signore M. Recent advances in the management of acute intracerebral hemorrhage. Curr Neurol Neurosci Rep. 2005;5(6):483–7.
van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007;369(9558):306–18.
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322.
CDC. http://www.cdc.gov/Stroke/faqs.htm. 2016.
Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of stroke during 1990–2010: findings from the global burden of disease study 2010. Lancet. 2014;383(9913):245–54.
Suwanwela NC, Poungvarin N. Asian stroke advisory P. Stroke burden and stroke care system in Asia. Neurol India. 2016;64(Suppl):S46–51.
Roth GA, Forouzanfar MH, Moran AE, Barber R, Nguyen G, Feigin VL, et al. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med. 2015;372(14):1333–41.
CLEAN M. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=1804.
EXTEND-IA. https://clinicaltrials.gov/ct2/show/NCT01492725.
Bendszus M, Thomalla G, Hacke W, Knauth M, Gerloff C, Bonekamp S, et al. Early termination of THRILL, a prospective study of mechanical thrombectomy in patients with acute ischemic stroke ineligible for i.V. Thrombolysis. Clin Neuroradiol. 2016; doi:10.1007/s00062-016-0538-8.
DAWN. https://www.clinicaltrialsgov/ct2/show/NCT02142283?term = DAWN&rank = 10.
POSITIVE. https://www.clinicaltrialsgov/ct2/show/NCT01852201?term = nct01852201&rank = 1.
Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723–31.
Berkhemer OAFP, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11–20.
Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019–30.
Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009–18.
Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296–306.
Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372(24):2285–95.
Rebello LC, Haussen DC, Grossberg JA, Belagaje S, Lima A, Anderson A, et al. Early endovascular treatment in intravenous tissue plasminogen activator-ineligible patients. Stroke. 2016;47(4):1131–4.
Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, et al. 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early Management of Patients with Acute Ischemic Stroke Regarding Endovascular Treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(10):3020–35.
Xie X, Lambrinos A, Chan B, Dhalla IA, Krings T, Casaubon LK, et al. Mechanical thrombectomy in patients with acute ischemic stroke: a cost-utility analysis. CMAJ Open. 2016;4(2):E316–25.
Aronsson M, Persson J, Blomstrand C, Wester P, Levin LA. Cost-effectiveness of endovascular thrombectomy in patients with acute ischemic stroke. Neurology. 2016;86(11):1053–9.
Mangla S, O'Connell K, Kumari D, Shahrzad M. Novel model of direct and indirect cost-benefit analysis of mechanical embolectomy over IV tPA for large vessel occlusions: a real-world dollar analysis based on improvements in mRS. J Neurointerv Surg. 2016; doi:10.1136/neurintsurg-2015-012152.
Lobotesis K, Veltkamp R, Carpenter IH, Claxton LM, Saver JL, Hodgson R. Cost-effectiveness of stent-retriever thrombectomy in combination with IV t-PA compared with IV t-PA alone for acute ischemic stroke in the UK. J Med Econ. 2016;19(8):785–94.
Health Quality Ontario. Mechanical thrombectomy in patients with acute ischemic stroke: a health technology assessment. Ont Health Technol Assess Ser. 2016;16(4):1–79.
Ganesalingam J, Pizzo E, Morris S, Sunderland T, Ames D, Lobotesis K. Cost-utility analysis of mechanical thrombectomy using stent retrievers in acute ischemic stroke. Stroke. 2015;46(9):2591–8.
Zivin JA, Simmons J. tPA for stroke : the story of a controversial drug. New York: Oxford University Press; 2011. xiv, 191 p. p.
Yamaguchi T, Mori E, Minematsu K, Nakagawara J, Hashi K, Saito I, et al. Alteplase at 0.6 mg/kg for acute ischemic stroke within 3 hours of onset: Japan alteplase clinical trial (J-ACT). Stroke. 2006;37(7):1810–5.
Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375(9727):1695–703.
Bluhmki E, Chamorro A, Davalos A, Machnig T, Sauce C, Wahlgren N, et al. Stroke treatment with alteplase given 3.0-4.5 h after onset of acute ischaemic stroke (ECASS III): additional outcomes and subgroup analysis of a randomised controlled trial. Lancet Neurol. 2009;8(12):1095–102.
NINDS. Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA stroke study group. N Engl J Med. 1995;333(24):1581–7.
Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317–29.
Lansberg MG, Bluhmki E, Thijs VN. Efficacy and safety of tissue plasminogen activator 3 to 4.5 hours after acute ischemic stroke: a metaanalysis. Stroke. 2009;40(7):2438–41.
Fang MC, Cutler DM, Rosen AB. Trends in thrombolytic use for ischemic stroke in the United States. J Hosp Med. 2010;5(7):406–9.
Reeves MJ, Arora S, Broderick JP, Frankel M, Heinrich JP, Hickenbottom S, et al. Acute stroke care in the US: results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry. Stroke. 2005;36(6):1232–40.
Schwamm LH, Ali SF, Reeves MJ, Smith EE, Saver JL, Messe S, et al. Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at get with the guidelines-stroke hospitals. Circ Cardiovasc Qual Outcomes. 2013;6(5):543–9.
Joo H, Wang G, George MG. Use of intravenous tissue plasminogen activator and hospital costs for patients with acute ischaemic stroke aged 18-64 years in the USA. Stroke Vasc Neurol. 2016;1(1):8–15.
Arora R, Salamon E, Katz JM, Cox M, Saver JL, Bhatt DL, et al. Use and outcomes of intravenous thrombolysis for acute ischemic stroke in patients >/=90 Years of age. Stroke. 2016;47(9):2347–54.
Taylor TN. The medical economics of stroke. Drugs. 1997;54(Suppl 3):51–7. discussion 7-8
Taylor TN, Davis PH, Torner JC, Holmes J, Meyer JW, Jacobson MF. Lifetime cost of stroke in the United States. Stroke. 1996;27(9):1459–66.
Boudreau DM, Guzauskas G, Villa KF, Fagan SC, Veenstra DL. A model of cost-effectiveness of tissue plasminogen activator in patient subgroups 3 to 4.5 hours after onset of acute ischemic stroke. Ann Emerg Med. 2013;61(1):46–55.
Boudreau DM, Guzauskas GF, Chen E, Lalla D, Tayama D, Fagan SC, et al. Cost-effectiveness of recombinant tissue-type plasminogen activator within 3 hours of acute ischemic stroke: current evidence. Stroke. 2014;45(10):3032–9.
Henninger N, Fisher M. Extending the time window for endovascular and pharmacological reperfusion. Transl Stroke Res. 2016;7(4):284–93.
Cassidy JM, Cramer SC. Spontaneous and therapeutic-induced mechanisms of functional recovery after stroke. Transl Stroke Res. 2016; doi:10.1007/s12975-016-0467-5.
Linfante I, Cipolla MJ. Improving reperfusion therapies in the era of mechanical thrombectomy. Transl Stroke Res. 2016;7(4):294–302.
Lyden P, Weymer S, Coffey C, Cudkowicz M, Berg S, O'Brien S, et al. Selecting patients for intra-arterial therapy in the context of a clinical trial for neuroprotection. Stroke. 2016;47(12):2979–85. doi:10.1161/STROKEAHA.116.013881.
Lapchak PA. Critical early Thrombolytic & Endovascular Reperfusion Therapy for Acute Ischemic Stroke Victims: a call for adjunct neuroprotection. Translational Stroke Research. 2015;6(5):345–54. doi:10.1007/s12975-015-0419-5(6):345-54.
Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999; 30(12):2752–8.
Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein R, Bradley EW, et al. A call for transparent reporting to optimize the predictive value of preclinical research. Nature. 2012;490(7419):187–91.
Lapchak PA. Scientific rigor recommendations for optimizing the clinical applicability of translational research. J Neurol Neurophysiol. 2012;3:e111.
Lapchak PA. Recommendations and practices to optimize stroke therapy: developing effective translational research programs. Stroke. 2013;44(3):841–3.
Crossley NA, Sena E, Goehler J, Horn J, van der Worp B, Bath PM, et al. Empirical evidence of bias in the design of experimental stroke studies: a metaepidemiologic approach. Stroke. 2008;39(3):929–34.
Hirst JA, Howick J, Aronson JK, Roberts N, Perera R, Koshiaris C, et al. The need for randomization in animal trials: an overview of systematic reviews. PLoS One. 2014;9(6):e98856.
Saver JL. Time is brain—quantified. Stroke. 2006;37(1):263–6.
Saver JL, Smith EE, Fonarow GC, Reeves MJ, Zhao X, Olson DM, et al. The "golden hour" and acute brain ischemia: presenting features and lytic therapy in >30,000 patients arriving within 60 minutes of stroke onset. Stroke. 2010;41(7):1431–9.
Lapchak PA. Fast neuroprotection (fast-NPRX) for acute ischemic stroke victims: the time for treatment is now. Transl Stroke Res. 2013;4(6):704–9.
Desai JA, Smith EE. Prenotification and other factors involved in rapid tPA administration. Curr Atheroscler Rep. 2013;15(7):337.
Olson DM, Constable M, Britz GW, Lin CB, Zimmer LO, Schwamm LH, et al. A qualitative assessment of practices associated with shorter door-to-needle time for thrombolytic therapy in acute ischemic stroke. J Neurosci Nurs. 2011;43(6):329–36.
Fonarow GC, Smith EE, Saver JL, Reeves MJ, Hernandez AF, Peterson ED, et al. Improving door-to-needle times in acute ischemic stroke: the design and rationale for the American Heart Association/American Stroke Association's target: stroke initiative. Stroke. 2011;42(10):2983–9.
Mokin M, Snyder KV, Siddiqui AH, Levy EI, Hopkins LN. Recent endovascular stroke trials and their impact on stroke Systems of Care. J Am Coll Cardiol. 2016;67(22):2645–55.
Carmichael ST. The 3 Rs of stroke biology: radial, relayed, and regenerative. Neurotherapeutics. 2016;13(2):348–59.
Lapchak PA. Drug-like property profiling of novel neuroprotective compounds to treat acute ischemic stroke: guidelines to develop pleiotropic molecules. Transl Stroke Res. 2013;4(3):328–42.
Lipinski CA. Drug-like properties and the causes of poor solubility and poor permeability. J Pharmacol Toxicol Methods. 2000;44(1):235–49.
Lipinski CA. Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol. 2004;1(4):337–41.
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46(1–3):3–26.
Hellinger E, Veszelka S, Toth AE, Walter F, Kittel A, Bakk ML, et al. Comparison of brain capillary endothelial cell-based and epithelial (MDCK-MDR1, Caco-2, and VB-Caco-2) cell-based surrogate blood-brain barrier penetration models. Eur J Pharm Biopharm. 2012;82(2):340–51.
Laine R. Metabolic stability: main enzymes involved and best tools to assess it. Curr Drug Metab. 2008;9(9):921–7.
Brimecombe JC, Kirsch GE, Brown AM. Test article concentrations in the hERG assay: losses through the perfusion, solubility and stability. J Pharmacol Toxicol Methods. 2009;59(1):29–34.
Cheng CS, Alderman D, Kwash J, Dessaint J, Patel R, Lescoe MK, et al. A high-throughput HERG potassium channel function assay: an old assay with a new look. Drug Dev Ind Pharm. 2002;28(2):177–91.
Gintant G. An evaluation of hERG current assay performance: translating preclinical safety studies to clinical QT prolongation. Pharmacol Ther. 2011;129(2):109–19.
Goineau S, Legrand C, Froget G. Whole-cell configuration of the patch-clamp technique in the hERG channel assay to predict the ability of a compound to prolong QT interval. Curr Protoc Pharmacol. 2012; Chapter 10:Unit 10 5.
Yu HB, Zou BY, Wang XL, Li M. Investigation of miscellaneous hERG inhibition in large diverse compound collection using automated patch-clamp assay. Acta Pharmacol Sin. 2016;37(1):111–23.
McDonnell AM, Dang CH. Basic review of the cytochrome p450 system. J Adv Pract Oncol. 2013;4(4):263–8.
Hedlund E, Gustafsson JA, Warner M. Cytochrome P450 in the brain: a review. Curr Drug Metab. 2001;2(3):245–63.
Evers R, Dallas S, Dickmann LJ, Fahmi OA, Kenny JR, Kraynov E, et al. Critical review of preclinical approaches to investigate cytochrome p450-mediated therapeutic protein drug-drug interactions and recommendations for best practices: a white paper. Drug Metab Dispos. 2013;41(9):1598–609.
Lapchak PA, Bombien R, Rajput PS. J-147 a Novel Hydrazide Lead Compound to Treat Neurodegeneration: CeeTox Safety and Genotoxicity Analysis. J Neurol Neurophysiol. 2013 ; 4(3).
Lapchak PA, Schubert DR, Maher PA. Delayed treatment with a novel neurotrophic compound reduces behavioral deficits in rabbit ischemic stroke. J Neurochem. 2011;116(1):122–31.
Kirsch-Volders M, Decordier I, Elhajouji A, Plas G, Aardema MJ, Fenech M. In vitro genotoxicity testing using the micronucleus assay in cell lines, human lymphocytes and 3D human skin models. Mutagenesis. 2011;26(1):177–84.
Wiesner J, Ziemann C, Hintz M, Reichenberg A, Ortmann R, Schlitzer M, et al. FR-900098, an antimalarial development candidate that inhibits the non-mevalonate isoprenoid biosynthesis pathway, shows no evidence of acute toxicity and genotoxicity. Virulence. 2016;7(6):718–28.
Steinmetz KL, Spack EG. The basics of preclinical drug development for neurodegenerative disease indications. BMC Neurol. 2009;9(Suppl 1):S2.
Pellegatti M. Preclinical in vivo ADME studies in drug development: a critical review. Expert Opin Drug Metab Toxicol. 2012;8(2):161–72.
Fisher M. Recommendations for advancing development of acute stroke therapies: stroke therapy academic industry roundtable 3. Stroke. 2003;34(6):1539–46.
Fisher M, Hanley DF, Howard G, Jauch EC, Warach S. Recommendations from the STAIR V meeting on acute stroke trials, technology and outcomes. Stroke. 2007;38(2):245–8.
Saver JL, Albers GW, Dunn B, Johnston KC, Fisher M, Consortium SV. Stroke therapy academic industry roundtable (STAIR) recommendations for extended window acute stroke therapy trials. Stroke. 2009;40(7):2594–600.
Albers GW, Goldstein LB, Hess DC, Wechsler LR, Furie KL, Gorelick PB, et al. Stroke treatment academic industry roundtable (STAIR) recommendations for maximizing the use of intravenous thrombolytics and expanding treatment options with intra-arterial and neuroprotective therapies. Stroke. 2011;42(9):2645–50.
Ahnstedt H, McCullough LD, Cipolla MJ. The importance of considering sex differences in translational stroke research. Transl Stroke Res. 2016;7(4):261–73.
Ergul A, Hafez S, Fouda A, Fagan SC. Impact of comorbidities on acute injury and recovery in preclinical stroke research: focus on hypertension and diabetes. Transl Stroke Res. 2016;7(4):248–60.
King AJ. The use of animal models in diabetes research. Br J Pharmacol. 2012;166(3):877–94.
Sasase T, Ohta T, Masuyama T, Yokoi N, Kakehashi A, Shinohara M. The spontaneously diabetic torii rat: an animal model of nonobese type 2 diabetes with severe diabetic complications. J Diabetes Res. 2013;2013:976209.
Kemmochi Y, Fukui K, Maki M, Kimura S, Ishii Y, Sasase T, et al. Metabolic disorders and diabetic complications in spontaneously diabetic Torii Lepr (fa) rat: a new obese type 2 diabetic model. J Diabetes Res. 2013;2013:948257.
Hossmann KA. The two pathophysiologies of focal brain ischemia: implications for translational stroke research. J Cereb Blood Flow Metab. 2012;32(7):1310–6.
Lapchak PA. A clinically relevant rabbit embolic stroke model for acute ischemic stroke therapy development: mechanisms & targets. In: Lapchak PA, Zhang JH, editors.: Translational Stroke Research: From Target Selection to Clinical Trials, Springer, USA; 2011. Chapter 27, p. 541–84
Lapchak PA. A cost-effective rabbit embolic stroke bioassay: insight into the development of acute ischemic stroke therapy. Transl Stroke Res. 2015;6(2):99–103.
Turner R, Jickling G. Sharp, F are underlying assumptions of current animal models of human stroke correct: from STAIRS to high hurdles? Translational Stroke Research. 2011;2(2):138–43.
Kent TA, Mandava P. Embracing biological and methodological variance in a new approach to pre-clinical stroke testing. Transl Stroke Res. 2016;7(4):274–83.
Lapchak PA, Chapman DF, Zivin JA. Metalloproteinase inhibition reduces thrombolytic (tissue plasminogen activator)-induced hemorrhage after thromboembolic stroke. Stroke. 2000;31(12):3034–40.
Liu DZ, Sharp FR. Excitatory and Mitogenic signaling in cell death, blood-brain barrier breakdown, and BBB repair after intracerebral hemorrhage. Transl Stroke Res. 2012;3(Suppl 1):62–9.
Turner RJ, Sharp FR. Implications of MMP9 for blood brain barrier disruption and hemorrhagic transformation following ischemic stroke. Front Cell Neurosci. 2016;10:56.
Kassner A, Merali Z. Assessment of blood-brain barrier disruption in stroke. Stroke. 2015;46(11):3310–5.
Borlongan CV. The blood brain barrier in stroke. Curr Pharm Des. 2012;18(25):3613–4.
Demaerschalk BM. Alteplase treatment in acute stroke: incorporating Food and Drug Administration prescribing information into existing acute stroke management guide. Curr Atheroscler Rep. 2016;18(8):53.
Benz RD. Toxicological and clinical computational analysis and the US FDA/CDER. Expert Opin Drug Metab Toxicol. 2007;3(1):109–24.
Muller PY, Milton M, Lloyd P, Sims J, Brennan FR. The minimum anticipated biological effect level (MABEL) for selection of first human dose in clinical trials with monoclonal antibodies. Curr Opin Biotechnol. 2009;20(6):722–9.
Sharma V, McNeill JH. To scale or not to scale: the principles of dose extrapolation. Br J Pharmacol. 2009;157(6):907–21.
Lapchak PA, Araujo DM, Zivin JA. Comparison of tenecteplase with alteplase on clinical rating scores following small clot embolic strokes in rabbits. Exp Neurol. 2004;185(1):154–9.
Lapchak PA, Daley JT, Boitano PD. A blinded, randomized study of L-arginine in small clot embolized rabbits. Exp Neurol. 2015;266:143–6.
Meador V, Jordan W, Zimmermann J. Increasing throughput in lead optimization in vivo toxicity screens. Curr Opin Drug Discov Devel. 2002;5(1):72–8.
Yoon M, Campbell JL, Andersen ME, Clewell HJ. Quantitative in vitro to in vivo extrapolation of cell-based toxicity assay results. Crit Rev Toxicol. 2012;42(8):633–52.
Lee SR, Wang X, Tsuji K, Lo EH. Extracellular proteolytic pathophysiology in the neurovascular unit after stroke. Neurol Res. 2004;26(8):854–61.
Richardson J, Murray D, House CK, Lowenkopf T. Successful implementation of the National Institutes of Health stroke scale on a stroke/neurovascular unit. J Neurosci Nurs. 2006;38(4 Suppl):309–15.
del Zoppo GJ. The neurovascular unit in the setting of stroke. J Intern Med. 2010;267(2):156–71.
Ago T. The neurovascular unit in health and ischemic stroke. Nihon Rinsho. 2016;74(4):583–8.
Cai W, Zhang K, Li P, Zhu L, Xu J, Yang B, et al. Dysfunction of the neurovascular unit in ischemic stroke and neurodegenerative diseases: An aging effect. Ageing Res Rev 2016.
ElAli A. The implication of neurovascular unit signaling in controlling the subtle balance between injury and repair following ischemic stroke. Neural Regen Res. 2016;11(6):914–5.
Lake EM, Bazzigaluppi P, Mester J, Thomason LA, Janik R, Brown M, et al. Neurovascular unit remodelling in the subacute stage of stroke recovery. Neuroimage. 2016. S1053-8119(16)30480-3. doi:10.1016/j.neuroimage.2016.09.016.
Hermann DM, Buga AM, Popa-Wagner A. Neurovascular remodeling in the aged ischemic brain. J Neural Transm (Vienna). 2015;122(Suppl 1):S25–33.
del Zoppo GJ. Aging and the neurovascular unit. Ann N Y Acad Sci. 2012;1268:127–33.
Redzic ZB, Rabie T, Sutherland BA, Buchan AM. Differential effects of paracrine factors on the survival of cells of the neurovascular unit during oxygen glucose deprivation. Int J Stroke. 2015;10(3):407–14.
Barakat R, Redzic Z. Differential cytokine expression by brain microglia/macrophages in primary culture after oxygen glucose deprivation and their protective effects on astrocytes during anoxia. Fluids Barriers CNS. 2015;12:6.
Dirnagl U. Pathobiology of injury after stroke: the neurovascular unit and beyond. Ann N Y Acad Sci. 2012;1268:21–5.
Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22(9):391–7.
Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67(2):181–98.
Xing C, Hayakawa K, Lo EH. Mechanisms, Imaging, and Therapy in Stroke Recovery. Transl Stroke Res. 2016. doi:10.1007/s12975-016-0503-5.
Jolkkonen J, Kwakkel G. Translational hurdles in stroke recovery studies. Transl Stroke Res. 2016;7(4):331–42.
Koh SH, Park HH. Neurogenesis in Stroke Recovery. Transl Stroke Res. 2016. doi:10.1007/s12975-016-0460-z.
Commercial sources were utilized to estimate assay costs. Sources include Cedars-Sinai Medical Center Department of Comparative Medicine, Pharmaron Inc, Absorption Systems Inc, Ricera inc., Cyprotex Inc, and Oxygen BioResearch PVT Ltd.
Acknowledgments and Funding
This article was written without direct financial support from government sources (PAL). JHZ was supported by NIH (NS081740 and NS084921).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
PAL is Editor-in-Chief, Journal of Neurology & Neurophysiology and Associate Editor, Translational Stroke Research; JHZ is Editor-in-Chief, Translational Stroke Research and Editor-in-Chief, Medical Gas Research.
Rights and permissions
About this article
Cite this article
Lapchak, P.A., Zhang, J.H. The High Cost of Stroke and Stroke Cytoprotection Research. Transl. Stroke Res. 8, 307–317 (2017). https://doi.org/10.1007/s12975-016-0518-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-016-0518-y