Abstract
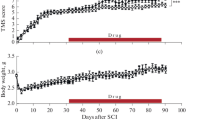
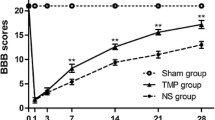
To assess whether phospholipase A2 (PLA2) plays a role in the pathogenesis of spinal cord injury (SCI), we compared lesions either induced by PLA2 alone or by a contusive SCI. At 24-h post-injury, both methods induced a focal hemorrhagic pathology. The PLA2 injury was mainly confined within the ventrolateral white matter, whereas the contusion injury widely affected both the gray and white matter. A prominent difference between the two models was that PLA2 induced a massive demyelination with axons remaining in the lesion area, whereas the contusion injury induced axonal damage and myelin breakdown. At 4 weeks, no cavitation was found within the PLA2 lesion, and numerous axons were myelinated by host-migrated Schwann cells. Among them, 45% of animals had early transcranial magnetic motor-evoked potential (tcMMEP) responses. In contrast, the contusive SCI induced a typical centralized cavity with reactive astrocytes forming a glial border. Only 15% of rats had early tcMMEP responses after the contusion. BBB scores were similarly reduced in both models. Our study indicates that PLA2 may play a unique role in mediating secondary SCI likely by targeting glial cells, particularly those of oligodendrocytes. This lesion model could also be used for studying demyelination and remyelination in the injured spinal cord associated with PLA2-mediated secondary SCI.






Similar content being viewed by others
References
Young W. Secondary injury mechanisms in acute spinal cord injury. J Emerg Med. 1993;11 Suppl 1:13–22.
Hall ED, Braughler JM. Role of lipid peroxidation in post-traumatic spinal cord degeneration: a review. Cent Nerv Syst Trauma. 1986;3(4):281–94.
Park E, Velumian AA, Fehlings MG. The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma. 2004;21(6):754–74.
Buki A, Okonkwo DO, Wang KK, Povlishock JT. Cytochrome c release and caspase activation in traumatic axonal injury. J Neurosci. 2000;20(8):2825–34.
Murakami M, Nakatani Y, Atsumi G, Inoue K, Kudo I. Regulatory functions of phospholipase A2. Crit Rev Immunol. 1997;17(3–4):225–83.
Farooqui AA, Yang HC, Rosenberger TA, Horrocks LA. Phospholipase A2 and its role in brain tissue. J Neurochem. 1997;69(3):889–901.
Bonventre JV. Roles of phospholipases A2 in brain cell and tissue injury associated with ischemia and excitotoxicity. J Lipid Mediat Cell Signal. 1996;14(1–3):15–23.
Farooqui AA, Litsky ML, Farooqui T, Horrocks LA. Inhibitors of intracellular phospholipase A2 activity: their neurochemical effects and therapeutical importance for neurological disorders. Brain Res Bull. 1999;49(3):139–53.
Klein J. Membrane breakdown in acute and chronic neurodegeneration: focus on choline-containing phospholipids. J Neural Transm. 2000;107(8–9):1027–63.
Beck S, Lambeau G, Scholz-Pedretti K, Gelb MH, Janssen MJ, Edwards SH, et al. Potentiation of tumor necrosis factor alpha-induced secreted phospholipase A2 (sPLA2)-IIA expression in mesangial cells by an autocrine loop involving sPLA2 and peroxisome proliferator-activated receptor alpha activation. J Biol Chem. 2003;278(32):29799–812.
van Rossum GS, Drummen GP, Verkleij AJ, Post JA, Boonstra J. Activation of cytosolic phospholipase A2 in Her14 fibroblasts by hydrogen peroxide: a p42/44(MAPK)-dependent and phosphorylation-independent mechanism. Biochim Biophys Acta. 2004;1636(2–3):183–95.
Kim DK, Rordorf G, Nemenoff RA, Koroshetz WJ, Bonventre JV. Glutamate stably enhances the activity of two cytosolic forms of phospholipase A2 in brain cortical cultures. Biochem J. 1995;310(Pt 1):83–90.
Sandhya TL, Ong WY, Horrocks LA, Farooqui AA. A light and electron microscopic study of cytoplasmic phospholipase A2 and cyclooxygenase-2 in the hippocampus after kainate lesions. Brain Res. 1998;788(1–2):223–31.
Clapp LE, Klette KL, DeCoster MA, Bernton E, Petras JM, Dave JR, et al. Phospholipase A2-induced neurotoxicity in vitro and in vivo in rats. Brain Res. 1995;693(1–2):101–11.
Kishimoto K, Matsumura K, Kataoka Y, Morii H, Watanabe Y. Localization of cytosolic phospholipase A2 messenger RNA mainly in neurons in the rat brain. Neuroscience. 1999;92(3):1061–77.
Molloy GY, Rattray M, Williams RJ. Genes encoding multiple forms of phospholipase A2 are expressed in rat brain. Neurosci Lett. 1998;258(3):139–42.
Ong WY, Horrocks LA, Farooqui AA. Immunocytochemical localization of cPLA2 in rat and monkey spinal cord. J Mol Neurosci. 1999;12(2):123–30.
Liu NK, Zhang YP, Titsworth WL, Jiang X, Han S, Lu PH, et al. A novel role of phospholipase A2 in mediating spinal cord secondary injury. Ann Neurol. 2006;59(4):606–19.
Titsworth WL, Onifer SM, Liu NK, Xu XM. Focal phospholipases A2 group III injections induce cervical white matter injury and functional deficits with delayed recovery concomitant with Schwann cell remyelination. Exp Neurol. 2007;207(1):150–62.
Liu N, Han S, Lu PH, Xu XM. Upregulation of annexins I, II, and V after traumatic spinal cord injury in adult rats. J Neurosci Res. 2004;77(3):391–401.
Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78(3):206–9.
Taoka Y, Okajima K, Uchiba M, Murakami K, Kushimoto S, Johno M, et al. Role of neutrophils in spinal cord injury in the rat. Neuroscience. 1997;79(4):1177–82.
Liu NK, Zhang YP, Han S, Pei J, Xu LY, Lu PH, et al. Annexin A1 reduces inflammatory reaction and tissue damage through inhibition of phospholipase A2 activation in adult rats following spinal cord injury. J Neuropathol Exp Neurol. 2007;66(10):932–43.
Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139(2):244–56.
Basso DM, Beattie MS, Bresnahan JC, Anderson DK, Faden AI, Gruner JA, et al. MASCIS evaluation of open field locomotor scores: effects of experience and teamwork on reliability. Multicenter Animal Spinal Cord Injury Study. J Neurotrauma. 1996;13(7):343–59.
Hall SM. The effect of injections of lysophosphatidyl choline into white matter of the adult mouse spinal cord. J Cell Sci. 1972;10(2):535–46.
Jeffery ND, Blakemore WF. Remyelination of mouse spinal cord axons demyelinated by local injection of lysolecithin. J Neurocytol. 1995;24(10):775–81.
Ousman SS, David S. Lysophosphatidylcholine induces rapid recruitment and activation of macrophages in the adult mouse spinal cord. Glia. 2000;30(1):92–104.
De S, Trigueros MA, Kalyvas A, David S. Phospholipase A2 plays an important role in myelin breakdown and phagocytosis during Wallerian degeneration. Mol Cell Neurosci. 2003;24(3):753–65.
Bresnahan JC. An electron-microscopic analysis of axonal alterations following blunt contusion of the spinal cord of the rhesus monkey (Macaca mulatta). J Neurol Sci. 1978;37(1–2):59–82.
Blakemore WF. Remyelination by Schwann cells of axons demyelinated by intraspinal injection of 6-aminonicotinamide in the rat. J Neurocytol. 1975;4(6):745–57.
Blakemore WF. The case for a central nervous system (CNS) origin for the Schwann cells that remyelinate CNS axons following concurrent loss of oligodendrocytes and astrocytes. Neuropathol Appl Neurobiol. 2005;31(1):1–10.
Griffin JW, Drucker N, Gold BG, Rosenfeld J, Benzaquen M, Charnas LR, et al. Schwann cell proliferation and migration during paranodal demyelination. J Neurosci. 1987;7(3):682–99.
Blakemore WF, Patterson RC. Observations on the interactions of Schwann cells and astrocytes following X-irradiation of neonatal rat spinal cord. J Neurocytol. 1975;4(5):573–85.
Talbott JF, Cao Q, Enzmann GU, Benton RL, Achim V, Cheng XX, et al. Schwann cell-like differentiation by adult oligodendrocyte precursor cells following engraftment into the demyelinated spinal cord is BMP-dependent. Glia. 2006;54(3):147–59.
Xu XM, Chen A, Guenard V, Kleitman N, Bunge MB. Bridging Schwann cell transplants promote axonal regeneration from both the rostral and caudal stumps of transected adult rat spinal cord. J Neurocytol. 1997;26(1):1–16.
Xu XM, Guenard V, Kleitman N, Bunge MB. Axonal regeneration into Schwann cell-seeded guidance channels grafted into transected adult rat spinal cord. J Comp Neurol. 1995;351(1):145–60.
Xu XM, Zhang SX, Li H, Aebischer P, Bunge MB. Regrowth of axons into the distal spinal cord through a Schwann-cell-seeded mini-channel implanted into hemisected adult rat spinal cord. Eur J Neurosci. 1999;11(5):1723–40.
Takami T, Oudega M, Bates ML, Wood PM, Kleitman N, Bunge MB. Schwann cell but not olfactory ensheathing glia transplants improve hindlimb locomotor performance in the moderately contused adult rat thoracic spinal cord. J Neurosci. 2002;22(15):6670–81.
Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, Filbin MT, et al. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004;10(6):610–6.
Cao Q, Zhang YP, Iannotti C, DeVries WH, Xu XM, Shields CB, et al. Functional and electrophysiological changes after graded traumatic spinal cord injury in adult rat. Exp Neurol. 2005;191 Suppl 1:S3–S16.
Farooqui AA, Ong WY, Horrocks LA. Biochemical aspects of neurodegeneration in human brain: involvement of neural membrane phospholipids and phospholipases A2. Neurochem Res. 2004;29(11):1961–77.
Toborek M, Malecki A, Garrido R, Mattson MP, Hennig B, Young B. Arachidonic acid-induced oxidative injury to cultured spinal cord neurons. J Neurochem. 1999;73(2):684–92.
Lu XR, Ong WY, Halliwell B. The phospholipase A2 inhibitor quinacrine prevents increased immunoreactivity to cytoplasmic phospholipase A2 (cPLA2) and hydroxynonenal (HNE) in neurons of the lateral septum following fimbria-fornix transection. Exp Brain Res. 2001;138(4):500–8.
O'Regan MH, Smith-Barbour M, Perkins LM, Phillis JW. A possible role for phospholipases in the release of neurotransmitter amino acids from ischemic rat cerebral cortex. Neurosci Lett. 1995;185(3):191–4.
Sundstrom E, Mo LL. Mechanisms of glutamate release in the rat spinal cord slices during metabolic inhibition. J Neurotrauma. 2002;19(2):257–66.
Phillis JW, O'Regan MH. Mechanisms of glutamate and aspartate release in the ischemic rat cerebral cortex. Brain Res. 1996;730(1–2):150–64.
Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med. 1997;3(1):73–6.
Liu XZ, Xu XM, Hu R, Du C, Zhang SX, McDonald JW, et al. Neuronal and glial apoptosis after traumatic spinal cord injury. J Neurosci. 1997;17(14):5395–406.
Beattie MS, Farooqui AA, Bresnahan JC. Review of current evidence for apoptosis after spinal cord injury. J Neurotrauma. 2000;17(10):915–25.
Warden P, Bamber NI, Li H, Esposito A, Ahmad KA, Hsu CY, et al. Delayed glial cell death following wallerian degeneration in white matter tracts after spinal cord dorsal column cordotomy in adult rats. Exp Neurol. 2001;168(2):213–24.
Cummings BS, McHowat J, Schnellmann RG. Phospholipase A(2)s in cell injury and death. J Pharmacol Exp Ther. 2000;294(3):793–9.
Taketo MM, Sonoshita M. Phospolipase A2 and apoptosis. Biochim Biophys Acta. 2002;1585(2–3):72–6.
Balsinde J, Perez R, Balboa MA. Calcium-independent phospholipase A2 and apoptosis. Biochim Biophys Acta. 2006;1761(11):1344–50.
Acknowledgments
We are thankful to Christine Nunn and Aaron Puckett for care of animals, and Darlene Burke and Kim Fentress for behavioral and electrophysiological assessments. This work was supported by NIH NS36350, NS52290, NS50243, NS059622, the Kentucky Spinal Cord and Head Injury Research Trust #4-16, the Daniel Heumann Fund for Spinal Cord Research, and Mari Hulman George Endowment Funds (XMX), and the Paralysis Project of America and the State of Indiana (Grant # 91910 and 91913; NKL).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, NK., Titsworth, W.L., Zhang, Y.P. et al. Characterizing Phospholipase A2-Induced Spinal Cord Injury—A Comparison with Contusive Spinal Cord Injury in Adult Rats. Transl. Stroke Res. 2, 608–618 (2011). https://doi.org/10.1007/s12975-011-0089-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-011-0089-x