Abstract
Ultra-thin strut polymer-free sirolimus-eluting stent (UPF-SES) have two novel characteristics, ultra-thin strut and polymer-free coating, which have the potential to achieve early re-endotherialization. However, a little is known whether early vascular healing of UPF-SES can be achieved in patients with acute coronary syndrome (ACS). The aim of this study was to evaluate the vascular healing after an implantation of UPF-SES in patients with ACS using optical coherence tomography (OCT) at 3 months after the stent implantation. From September 2020 and January 2021, a total of 31 consecutive patients presenting with ACS who underwent OCT-guided percutaneous coronary intervention (PCI) and 3 month follow-up OCT examination were enrolled in the USUI-ACS study. The endpoints of this study were neointimal strut coverage, malapposition, and mean neointimal hyperplasia (NIH) thickness at 3 month follow-up. Over a mean follow-up of 91 days after the initial PCI, the follow-up OCT was examined. The median percentage of covered struts was 98.4% and malapposed struts 0%, and the mean NIH thickness was 80 μm. UPF-SES exhibited an excellent early vascular healing at 3 months in patients with ACS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As compared to bare metal stents, first generation drug-eluting stents (DESs) have dramatically reduced the incidence of restenosis and target lesion revascularization by inhibiting neointimal hyperplasia [1]. However, there remains a concern about the increasing risk of late stent thrombosis related to incomplete endothelialization and delayed healing [2, 3]. Therefore, new generation DESs have been designed to overcome these limitations by using different antiproliferative drugs and release kinetics, thinner and improved stent struts, and the development of biocompatible, bioabsorbable polymer coatings or a polymer-free technology [4]. Ultra-thin strut polymer-free sirolimus-eluting stent (UPF-SES) (Coroflex ISAR NEO; B Braun Melsungen AG, Melsungen, Germany) is a newly developed DES that consist of ultra-thin struts (55 µm for less than 3 mm stents and 65 µm for 3.5–4.0 mm stents) with a dual drug coating of probucol and sirolimus without a polymer. As a hypothesis, ultra-thin struts cause less vessel injury than thicker struts, and a non-polymer coating avoids local arterial hypersensitivity and chronic inflammatory reactions. Therefore, UPF-SES can achieve early vascular healing after the stent implantation. Actually, FRIENDLY OCT trial compared UPF-SES (Coroflex ISAR; B. Braun Melsungen AG, Melsungen, Germany), which consist of ultra-thin struts (50 μm for less than 2.5 mm and 60 μm for more than 2.75 mm) and a dual drug coating (probucol and sirolimus) without a polymer, with thin strut bioabsorbable polymer coating SES (BP-SES) (Ultimaster; Termo Corporation, Tokyo, Japan) at the 3 month follow-up optical coherence tomography (OCT) [5]. The study demonstrated that UPF-SES had earlier and excellent vascular healing than BP-SES at 3 months. However, the limitations of the study were exclusion of ST-segment elevation myocardial infarction (STEMI) patients and inclusion of high proportion of non-acute coronary syndrome (ACS) patients (44%) [5]. Therefore, a little is known whether early vascular healing of UPF-SES can be achieved even in ACS patients.
We conducted a prospective observational study to evaluate whether early vascular healing of UPF-SES can be achieved in patients with ACS using 3 month follow-up OCT examination.
Methods
Patient population
We prospectively enrolled consecutive patients who underwent percutaneous coronary intervention (PCI) in our institution from September 2020 to January 2021. All the patients were registered in the early vascular healing of Ultra-thin Strut polymer-free sirolimus-elutIng stents in Acute Coronary Syndrome (USUI-ACS) registry. The inclusion criteria were as follows: (1) patients presenting with ACS [STEMI, non-ST-segment elevation myocardial infarction (NSTEMI), and unstable angina (UA)]; (2) de novo lesion with > 50% diameter stenosis; (3) patients who underwent OCT-guided PCI; and 4) patients who agreed to follow-up angiographic and OCT examination at 3 month. The exclusion criteria were as follows: (1) a left main lesion, right ostial or coronary bypass graft lesion; (2) in-stent restenosis; and (3) chronic kidney disease defined as a serum creatinine level > 2.0 mg/dl or maintenance hemodialysis. A loading dose of aspirin (81–200 mg) and prasugrel (20 mg) (the standard loading dose in Japan) was administered before the PCI. Dual antiplatelet therapy (DAPT) was recommended in accordance with the Japanese Circulation Society guidelines [6]. Written informed consent to participation was obtained from all patients in accordance with the Declaration of Helsinki, and the protocol was approved by the Osaka Rosai Hospital ethics committees. It was registered under the Japanese UMIN Clinical Trial Registration (UMIN 000,042,271).
Procedural management
PCI was performed according to standard techniques. Radial access is recommended over femoral access [7]. The direct stenting, thrombectomy, pre-dilatation, and post dilatation were left to each operator’s discretion, and the operator referred to the OCT findings to determine the strategy. The stent diameter was basically determined according to the external elastic lamina (EEL)-based approach [8]. The reference site was set at a cross-section adjacent to the lesion that is most normal looking and free of lipid plaque [9]. We measured proximal and distal reference mean EEL diameters and used the smaller of them rounded down to the nearest 0.25 mm to determine stent diameter [8]. Stent length was selected by measuring distance from the distal reference to the proximal reference. Post dilatation was recommended when malapposition ≥ 400 μm was observed [10].
Study endpoints
The primary endpoint was the percentage of covered struts at the 3 month follow-up. Secondary endpoints were the other OCT findings at the 3 month follow-up including the percentage of malapposed struts and mean neointimal hyperplasia (NIH) thickness. The co-secondary endpoints were the incidence of cardiac death, target vessel myocardial infarction, ischemia driven target lesion revascularization, and stent thrombosis within one year. Cardiac death was defined as any death due to proximate cardiac cause, unwitnessed death and death of unknown cause, all procedure related deaths [11]. Myocardial infarction was defined as a characteristics rise and fall of creatinine kinase-MB fraction or troponin in the presence of at least one of ischemic symptoms, new pathological Q wave, ischemic electrocardiographic changes, and pathological evidence of acute myocardial infarction [12]. Target lesion revascularization was defined as repeat PCI or bypass graft placement for restenosis or other complications at the lesion treated during index PCI, or occurring within 5 mm of the PCI site [11]. Stent thrombosis was categorized according to the definitions provided by the Academic Research Consortium [11].
OCT image acquisition
OCT images were acquired with OPTIS MOBILE OCT system (Abbott Vascular, Santa Clara, CA, USA) after the administration of intracoronary nitroglycerin. A conventional angioplasty guidewire (0.014 inch) was advanced distal to the region of interest, and then the OCT imaging catheter (Dragonfly, Abbott Vascular, Santa Clara, CA, USA) was advanced over the guidewire beyond the region of interest. During the imaging acquisition, blood was displaced by an injection of contrast media. The images were calibrated by an automated adjustment of the Z-offset and the automated pullback was set at 18 or 36 mm/s.
OCT image analysis
We performed quantitative and qualitative OCT analyses using dedicated software (Off-line Review Workstation, version E.0.2, Abbott Vascular, Santa Clara, CA, USA). We previously published the details of the OCT analysis [13]. All variables were analyzed at lesion level. In brief, the cross-sectional OCT images were analyzed at 1-mm intervals within the entire target segment of the pre-procedural, post-procedural, and 3 month follow-up OCT data. In the pre-procedural OCT data, lesion morphology was assessed at the culprit site and the lesions were categorized as follow: plaque rupture, plaque erosion, calcified nodule or unknown [9]. Minimum lumen area was derived from an automatic lumen segmentation within the region of interest. In the post-procedural OCT data, minimum lumen area was derived from an automatic lumen segmentation within the stented lesion. Stent area tracings were manually performed at 1-mm intervals within the stented lesion and minimum stent area within the stented lesion was evaluated. In-stent tissue protrusions were divided into three categories: smooth protrusions, disrupted fibrous tissue protrusion, and irregular protrusions [13]. In the follow-up OCT data, struts were classified as uncovered if any part of the strut was visibly exposed to the lumen and as covered if a layer of tissue was visible over all the reflecting surfaces. A malapposed strut was defined as a distance between the center of the strut blooming into the luminal contour of the arterial wall of > 55 µm for less than 3 mm stents and > 65 µm for 3.5–4.0 mm stents. The percentage of covered and malapposed struts were calculated as the number of covered and malapposed struts divided by the total number of analyzed struts for each lesion. The NIH thickness was defined as the distance between the endoluminal surface of the strut reflection and the lumen contour. Mean NIH thickness (total NIH thickness divided by total struts) was calculated for each lesion. The inter-observer reproducibility of the OCT image analysis in our institution were shown in a previous article [13].
Statistical analysis
No sample size calculation was performed due to the absence of previous data. Categorical variables are stated as numbers (percentages) and compared using a Pearson’s chi-squared test. Continuous variables are stated as the mean ± standard deviation or median (interquartile range) and compared using a Student’s t-test and the Mann–Whitney U test, based on the distribution. A p value < 0.05 was considered significant. We also compared the pre-procedural, post-procedural and follow-up OCT data between STEMI and NSTEMI/UA for the exploratory analysis. The statistical analysis was performed using the R programming language and environment version 3.6.1.
Results
Study population
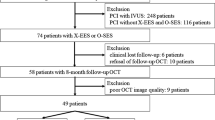
Figure 1 shows the study flow chart. From September 2020 to January 2021, a total of 31 consecutive patients were enrolled in the present study. 25 ineligible patients were treated with intravascular ultrasound (IVUS) mainly due to chronic kidney disease. 3 patients were lost to follow-up due to COVID-19 pandemic, and one patient was due to the progression of cancer and the other patient was due to the progression of interstitial pneumonia. There was no patient who had any cardiac events including stent thrombosis during the 3 months.
Study Flow Chart. Sixty-one consecutive patients presenting with acute coronary syndrome (ACS) who underwent percutaneous coronary intervention (PCI) were assessed for eligibility in our institution between September 2020 and January 2021. In twenty-five patients who were excluded from the study due to intravascular ultrasound guided PCI, eight patients had chronic kidney disease (CKD) or hemodialysis (HD). Thirty-five patients underwent PCI using optical coherence tomography (OCT) and ultra-thin strut polymer-free sirolimus eluting stent (UPF-SES) were implanted. Finally, a total of thirty-one patients underwent a 3 month follow-up OCT examination
Patient, lesion, and procedural characteristics
The baseline patient, lesion, and procedural characteristics are summarized in Table 1. The proportion of STEMIs was 61%, NSTEMI 26%, and UA 13%. The median stent size and length were 3.00 mm and 28 mm, respectively. All patients had DAPT for 3 months.
OCT findings at pre and post procedure
Only one pre-procedure OCT pull-back image was excluded because of a poor image quality. All the other OCT pull-back images were analyzed. Table 2 shows the pre-procedural and post-procedural OCT findings. White or red thrombus was observed in 63% and plaque rupture in 67%. The minimum stent area was 5.66 mm2. The percentage of malapposed struts was 2.3% and proportion of irregular protrusion 61%.
Three-month follow-up OCT findings
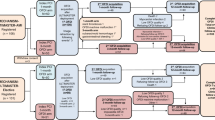
The mean follow-up OCT analysis was performed 91 days after the initial PCI. The follow-up OCT findings are presented in Table 3. The percentage of covered struts was 98.4% and malapposed struts 0%, and the mean NIH thickness was 80 μm. Figure 2 shows the individual serial changes in the percentage of malapposed struts. The percentage of malapposed struts significantly decreased during the 3 month follow-up period (p < 0.01). Figure 3 demonstrates the representative OCT images of the struts and neointimal coverage over 3 month. The comparison of the OCT findings between STEMI and NSTEMI/UA was shown in Table 4. At the 3 month follow-up OCT data, the percentage of covered struts did not differ between the two groups. The percentage of malapposed struts was significantly higher in STEMI than in NSTEMI/UA, but the absolute difference was low.
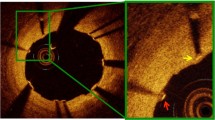
Representative Serial OCT Images of the Struts and Neointimal Coverage over 3 months. Ultra-thin strut polymer-free stent (4.0 × 24 mm) was implanted in the patient. The left panel shows the distal site, middle panel the culprit site, and right panel the proximal site. All stent struts at those sites were covered by the neointima over 3 months
One-year clinical outcome
At 1 year of follow-up, cardiac death occurred in 1 (3%) patient due to heart failure. There was no target vessel myocardial infarction, ischemia driven target lesion revascularization and stent thrombosis (definite, probable, and possible) within 1 year.
Discussion
The present OCT study demonstrated excellent strut coverage and apposition of the UPF-SES at 3 month follow-up in patients with ACS. In addition, 1 year clinical outcomes were acceptable.
Early vascular healing of UPF-SES in patients with ACS
Concerning the pathology, it has been reported that vessel healing of DESs in patients with ACS is substantially delayed as compared to that in those with stable angina [14]. Actually, MECHANISM-ULTIMASTER study, the study evaluated the serial vascular healing of BP-SES, showed that the percentage of covered struts were significantly lower in STEMI patients than in non-ACS patients [15]. The recent OCT analysis of UPF-SES, FRIENDLY OCT trial, showed that the percentage of covered struts at 3 months was significantly higher in UPF-SES than BP-SES (96.89% vs. 94.65%; p < 0.001, respectively) in patients with non-ACS, NSTEMI and UA [5]. On the other hand, the present study showed the strut coverage of UPF-SES at 3 months in patients with only ACS including STEMI, NSTEMI and UA. Our data demonstrated that the strut coverage of UPF-SES in ACS patients was comparable to that of FRIENDLY OCT trial (98.4% vs. 96.89%, respectively) even though our study population had higher risk of delayed healing than the population of FRIENDLY OCT trial. Moreover, the comparison of the 3 month follow-up OCT findings in patients with ACS among the present study and several previously reported studies was shown in the Supplementary Table 1 [15,16,17]. The percentage of covered struts and malapposed struts in UPF-SES were comparable as compared to those of other recent DESs [15,16,17]. Of course, our sample size were very small and the patient population in the current study was completely different compared to the other studies mentioned above. Therefore, these observations need cautious interpretation but may indicate the efficacy of UPF-SES in patients with ACS.
Unique characteristics of UPF-SES
UPF-SES has two unique characteristics, ultra-thin strut and non-polymer coating, and we believe these two characteristics relate to early vascular healing after the stent implantation in patients with ACS. In the bare metal stent era, the ISAR-STEREO trial and ISAR-STEREO-2 trial revealed that ultra-thin struts are associated with more favorable healing than that of thick struts due to less vessel injury and inflammation [18, 19]. Another article has shown that low endothelial shear stress downstream of the thick strut promotes stent thrombogenicity and retards re-endothelialization [20]. Therefore, ultra-thin strut can promote earlier re-endothelialization than thicker strut. Furthermore, UPF-SES consists of a dual drug coating of probucol and sirolimus. The drugs were completely released about 3 months after the stent implantation due to this dual drug technology. This release kinetics was as same as other recent DESs. In a previous study, thick-strut polymer-free biolimus A9-coated stent (BioFreedom, Biosensors, Morges, Switzerland) failed to show the noninferiority for 1 year clinical outcomes as compared to ultra-thin strut bioabsorbable polymer coating SES (Orsiro; Biotronik, Bülach, Switzerland) [21]. The article discussed that fast drug release (1 month) and thick strut may have contributed to the higher event rate observed in the BioFreedom stent [21]. UPF-SES overcomes the two problems. On the other hand, durable polymer can cause a chronic inflammatory reaction and lead to impair re-endothelialization [22]. To overcome this problem, BP coatings have been developed and added to new generation DESs. Actually, our previous OCT study have demonstrated that BP coating have an earlier vascular healing than durable polymer (DP) coating in patients with non-ACS and ACS [13]. As a hypothesis, polymer-free stents can be free from polymer-related inflammation just after stent implantation and may promote earlier re-endothelialization than bioabsorbable polymer DESs. In summary, both ultra-thin strut and non-polymer coating can achieve early vascular healing.
Vascular healing in patients with STEMI
The current study included a high proportion of STEMI patients (61%). STEMI is one of the risk factors of delayed healing. A previous report demonstrated that thrombus was dissolved due to antiplatelet therapy after stent implantation in ACS which may induced stent malapposition [23]. In fact, our data showed that the percentage of malapposed struts was statistically higher in STEMI than in NSTEMI/UA, though the absolute difference was low. The higher incidence of red or white thrombus at the initial procedures in STEMI than in NSTEMI/UA might contribute to the result above mentioned. On the other hand, the percentage of covered struts was excellent in patients with STEMI. A previous OCT study demonstrated that BP-everolimus eluting stent (EES) was associated with better vascular healing than DP-EES at 4 months in patients with STEMI [24]. Accordingly, polymer was one of the important factors in terms of vascular healing in patients with STEMI. Therefore, we hypothesize that non-polymer coating in addition to ultra-thin struts might play one of the key roles in vascular healing specifically in the setting of STEMI.
OCT-guided PCI for ACS
Our OCT data showed the excellent stent coverage at 3 month follow-up. We believe that relatively low late of acute stent malapposition after the initial procedure due to OCT-guided PCI have contributed to the results. In fact, a recent OCT analysis demonstrated that acute stent apposition was associated with chronic stent coverage [25]. It is speculated that ACS are associated with high rate of acute incomplete stent apposition [26]. OCT imaging delivers a higher resolution than IVUS and can precisely detect stent apposition [9]. In TRANSFORM OCT trial, in which 70% patients were ACS and no recommendation for OCT-guided PCI was observed, acute stent malapposition was 4–5% detected by OCT [27]. In our previous retrospective OCT analysis, in which 30% patients were ACS and OCT-guided PCI with lumen-based approach was recommended, acute stent malapposition was about 3.5% [13]. In the present study, in which OCT-guided PCI with EEL-based approach was recommended, acute stent malapposition was 2.3%. ILUMIEN III trial showed the lower percentage of malapposition in OCT-guided PCI with EEL-based approach than in angiography-guided PCI [8]. The sub-analysis of the trial also revealed that EEL-based approach led to selection of larger stent diameter than lumen-based approach [28]. Moreover, the sub-analysis of MECHANISM study revealed that EEL-based approach was safe and feasible as compared to lumen-based approach in stable coronary artery disease [29]. However, there is still room for improvement in our OCT-guided PCI. In the current study, the number of post dilatation was relatively small (52%) and acute malapposition ≥ 400 μm was observed in 29% though we recommended post dilatation when malapposition ≥ 400 μm was observed. We believe that OCT-guided PCI can improve acute and early stent results in patients with ACS.
Clinical implications
ACS is a cornerstone of PCI even with the development of DESs. A high rate of target lesion failure including stent thrombosis occurs in ACS patients and stent thrombosis is occasionally relates to high mortality rate [30]. Previous OCT study has shown that the presence of uncovered and malapposed struts are the major causes of stent thrombosis [31]. Therefore, an alternative stent design, in which early and good strut coverage is observed, is necessary for ACS. Previous meta-analysis demonstrated ultra-thin strut DES as thin as UPF-SES were associated with qualitatively lower rates of stent thrombosis as compared to 2nd generation DES [32]. Therefore, we think UPF-SES may be one of the options for patients with ACS and we hope UPF-SES reduce target lesion failure including stent thrombosis. To the best of our knowledge, the present study was the first to show the excellent early vascular healing of UPF-SES in patients with ACS, and we believe our result suggest the need for a properly powered randomized comparison of vascular healing and clinical outcomes between UPF-SES and recent DESs in patients with ACS.
Limitations
The major limitations of the present study were single-arm non-control design and very small sample size. Second, we selected ACS patients who performed OCT examination at the initial procedure and agreed to perform 3 month follow-up OCT examination. OCT is generally unfavorable for patients with unstable hemodynamics, impaired renal function, and ostial lesion as compared to IVUS. Therefore, it has a risk of selection bias. Third, we did not show any further clinical benefit of UPF-SES over other recent DESs in the present study.
Conclusions
The results of the USUI-ACS study demonstrated that ultra-thin strut polymer-free sirolimus eluting stents had excellent vascular healing at the 3 month follow-up in patients with ACS.
References
Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O’Shaughnessy C, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349:1315–23.
Cook S, Wenaweser P, Togni M, Billinger M, Morger C, Seiler C, et al. Incomplete stent apposition and very late stent thrombosis after drug-eluting stent implantation. Circulation. 2007;115:2426–34.
Kuramitsu S, Sonoda S, Ando K, Otake H, Natsuaki M, Anai R, et al. Drug-eluting stent thrombosis: current and future perspectives. Cardiovasc Interv Ther. 2021;36:158–68.
Saito Y, Kobayashi Y. Contemporary coronary drug-eluting and coated stents: a mini-review. Cardiovasc Interv Ther. 2021;36:20–2.
Otaegui I, Pérez de Prado A, Massotti M, López-Benito M, Sabaté M, Martí G, et al. Intrapatient randomization to study strut coverage in polymer-free versus biodegradable-polymer sirolimus-eluting stent implantations. JACC Cardiovasc Interv. 2020;13:899–900.
Nakamura M, Kimura K, Kimura T, Ishihara M, Otsuka F, Kozuma K, et al. JCS 2020 guideline focused update on antithrombotic therapy in patients with coronary artery disease. Circ J. 2020;84:831–65.
Ozaki Y, Hara H, Onuma Y, Katagiri Y, Amano T, Kobayashi Y, et al. CVIT expert consensus document on primary percutaneous coronary intervention (PCI) for acute myocardial infarction (AMI) update 2022. Cardiovasc Interv Ther. 2022;37:1–34.
Ali ZA, Maehara A, Généreux P, Shlofmitz RA, Fabbiocchi F, Nazif TM, et al. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomised controlled trial. Lancet. 2016;388:2618–28.
Fujii K, Kubo T, Otake H, Nakazawa G, Sonoda S, Hibi K, et al. Expert consensus statement for quantitative measurement and morphological assessment of optical coherence tomography: update 2022. Cardiovasc Interv Ther. 2022;37:248–54.
Räber L, Mintz GS, Koskinas KC, Johnson TW, Holm NR, Onuma Y, et al. Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European association of percutaneous cardiovascular interventions. Eur Heart J. 2018;39:3281–300.
Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–51.
Garcia-Garcia HM, McFadden EP, Farb A, Mehran R, Stone GW, Spertus J, et al. Standardized end point definitions for coronary intervention trials: the academic research consortium-2 consensus document. Circulation. 2018;137:2635–50.
Matsuhiro Y, Nakamura D, Shutta R, Kawamura A, Nakamura H, Okamoto N, et al. Difference of vascular healing between bioabsorbable-polymer and durable-polymer new generation drug-eluting stents: an optical coherence tomographic analysis. Int J Cardiovasc Imaging. 2021;37:1131–41.
Nakazawa G, Finn AV, Joner M, Ladich E, Kutys R, Mont EK, et al. Delayed arterial healing and increased late stent thrombosis at culprit sites after drug-eluting stent placement for acute myocardial infarction patients: an autopsy study. Circulation. 2008;118:1138–45.
Itoh T, Otake H, Kimura T, Tsukiyama Y, Kikuchi T, Okubo M, et al. A serial optical frequency-domain imaging study of early and late vascular responses to bioresorbable-polymer sirolimus-eluting stents for the treatment of acute myocardial infarction and stable coronary artery disease patients: results of the Mechanism-Ultimaster study. Cardiovasc Interv Ther. 2021;37:281–92.
Ishida M, Terashita D, Itoh T, Otake H, Tsukiyama Y, Kikuchi T, et al. Vascular response occurring at 3 months after everolimus-eluting cobalt-chromium stent implantation in patients with ST-segment elevation myocardial infarction vs. stable coronary artery disease. Circ J: Off J Jpn Circ Soc. 2020;84:1941–8.
Karjalainen PP, Varho V, Nammas W, Mikkelsson J, Pietilä M, Ylitalo A, et al. Early neointimal coverage and vasodilator response following biodegradable polymer sirolimus-eluting vs. durable polymer zotarolimus-eluting stents in patients with acute coronary syndrome—HATTRICK-OCT trial. Circ J: Off J Jpn Circ Soc. 2015;79:360–7.
Kastrati A, Mehilli J, Dirschinger J, Dotzer F, Schühlen H, Neumann FJ, et al. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO) trial. Circulation. 2001;103:2816–21.
Pache J, Kastrati A, Mehilli J, Schühlen H, Dotzer F, Hausleiter J, et al. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO-2) trial. J Am Coll Cardiol. 2003;41:1283–8.
Kolandaivelu K, Swaminathan R, Gibson WJ, Kolachalama VB, Nguyen-Ehrenreich KL, Giddings VL, et al. Stent thrombogenicity early in high-risk interventional settings is driven by stent design and deployment and protected by polymer-drug coatings. Circulation. 2011;123:1400–9.
Jensen Lisette O, Maeng M, Raungaard B, Kahlert J, Ellert J, Jakobsen L, et al. Randomized comparison of the polymer-free biolimus-coated biofreedom stent with the ultrathin strut biodegradable polymer sirolimus-eluting orsiro stent in an all-comers population treated with percutaneous coronary intervention: the SORT OUT IX trial. Circulation. 2020;141:2052–63.
Virmani R, Guagliumi G, Farb A, Musumeci G, Grieco N, Motta T, et al. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious? Circulation. 2004;109:701–5.
Guo N, Maehara A, Mintz GS, He Y, Xu K, Wu X, et al. Incidence, mechanisms, predictors, and clinical impact of acute and late stent malapposition after primary intervention in patients with acute myocardial infarction: an intravascular ultrasound substudy of the harmonizing outcomes with revascularization and stents in acute myocardial infarction (HORIZONS-AMI) trial. Circulation. 2010;122:1077–84.
Shimoda M, Ando H, Naito K, Suzuki A, Sakurai S, Nakano Y, et al. Early-phase vascular healing of bioabsorbable vs. durable polymer-coated everolimus-eluting stents in patients with ST-elevation myocardial infarction—2 week and 4 month analyses with optical coherence tomography. Circ J: Off J Jpn Circ Soc. 2018;82:2594–601.
Noguchi M, Dohi T, Okazaki S, Matsumura M, Takeuchi M, Endo H, et al. Comparison of 6-month vascular healing response after bioresorbable polymer versus durable polymer drug-eluting stent implantation in patients with acute coronary syndromes: a randomized serial optical coherence tomography study. Catheter Cardiovasc Interv. 2021;98:E677–86.
Kurogi K, Ishii M, Yamamoto N, Yamanaga K, Tsujita K. Optical coherence tomography-guided percutaneous coronary intervention: a review of current clinical applications. Cardiovasc Interv Ther. 2021;36:169–77.
Guagliumi G, Shimamura K, Sirbu V, Garbo R, Boccuzzi G, Vassileva A, et al. Temporal course of vascular healing and neoatherosclerosis after implantation of durable- or biodegradable-polymer drug-eluting stents. Eur Heart J. 2018;39:2448–56.
Shlofmitz E, Jeremias A, Parviz Y, Karimi Galougahi K, Redfors B, Petrossian G, et al. External elastic lamina vs. luminal diameter measurement for determining stent diameter by optical coherence tomography: an ILUMIEN III substudy. Eur Heart J Cardiovasc Imaging. 2021;22:753–9.
Sasaki W, Ishida M, Itoh T, Uchimura Y, Oda H, Taguchi Y, et al. Comparison of serial optical coherence tomography imaging following aggressive stent expansion technique: insight from the MECHANISM study. Int J Cardiovasc Imaging. 2021;37:419–28.
Claessen BE, Henriques JP, Jaffer FA, Mehran R, Piek JJ, Dangas GD. Stent thrombosis: a clinical perspective. JACC Cardiovasc Interv. 2014;7:1081–92.
Taniwaki M, Radu MD, Zaugg S, Amabile N, Garcia-Garcia HM, Yamaji K, et al. Mechanisms of very late drug-eluting stent thrombosis assessed by optical coherence tomography. Circulation. 2016;133:650–60.
Bangalore S, Toklu B, Patel N, Feit F, Stone GW. Newer-generation ultrathin strut drug-eluting stents versus older second-generation thicker strut drug-eluting stents for coronary artery disease. Circulation. 2018;138:2216–26.
Acknowledgements
The authors thank Mr. John Martin for his linguistic assistance with this manuscript.
Funding
We have no funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All authors have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Matsuhiro, Y., Egami, Y., Okamoto, N. et al. Early vascular healing of ultra-thin strut polymer-free sirolimus-eluting stents in acute coronary syndrome: USUI-ACS study. Cardiovasc Interv and Ther 38, 55–63 (2023). https://doi.org/10.1007/s12928-022-00862-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12928-022-00862-2