Abstract
Risk of cancer especially of colon, breast, and pancreas is high in diabetic and obese patients, with potential involvement of augmented expression of RAGE (receptor for advanced glycation end products) and its ligands, namely AGEs (advanced glycation end products), HMGB1 (high-mobility group box 1 protein), and S100 group of proteins. Studies have reported the involvement of RAGE activation by its ligands in growth and survival of cancers, including metastasis and poor prognosis. We propose that this receptor-ligand axis provides the molecular link between certain pre-existing states as hypoxia, hyperglycemia, glycation, inflammation, oxidative stress, and onset of cancers. The chronic inflammatory, hyperglycemic milieu accompanied by glycoxidative stress as in diabetes and obesity, concomitant with the formation of RAGE ligands, instigates RAGE and cancer stem cells, leading to the oncogenic transformation of normal and pre-malignant tissues towards development of neoplasms. We have aimed to elucidate the complete signalling map initiated upon RAGE-ligand splicing, from oncogenesis to progression, epithelial-mesenchymal transition, invasion, cancer stem cell renewal, chemo-resistance, and cancer relapse. We have attributed the complex molecular functions of RAGE-ligand signalling cues to every aspect of cancer promotion, explaining the central network in bridging glycation, inflammation, oxidation, and the hallmarks of cancer. Underlining the substantial requisite for anti-neoplastic agents targeting RAGE and its ligands, we have explicitly discoursed RAGE and its allied components (AGEs, soluble RAGE, RAGE gene polymorphisms) as potential diagnostic and prognostic biomarkers for prompt detection of cancers and implication in impending RAGE-ligand directed, novel combinatorial, and targeted onco-therapeutics.
Similar content being viewed by others
Introduction
Neoplasm or cancer is the unrestricted growth, expansion, and spread of cells, triggered by stimulation of oncogenes and/or growth factors, abrogation of tumour suppressors and/or cell cycle check points, disparities in redox signalling with sequential indulgence of multiple genes and signalling pathways, characterised by deranged cellular architecture, and altered genomic plus metabolic signature. Cancer is the second leading cause of death globally, accounting for one in six deaths. It was responsible for 8.8 million deaths in 2015 [1].
Metabolic inclination of cancer cells towards an accelerated rate of aerobic glycolysis (Warburg’s hypothesis) in par with their uncontrolled proliferation (to meet energy requirements) creates a hyperglycemic microenvironment prone to oxidative stress and glycation, in addition to related inflammation [2, 3]. The by-products of enhanced glycolytic flux called advanced glycation end products (AGEs) have been implicated in the progression and invasion of cancers [4, 5]. Several studies have reported the presence of different types of protein-AGEs namely, argpyrimidine and carboxy methyl lysine [6] and DNA-AGEs like CEdG (N2-(1-carboxyethyl)-2′-deoxyguanosine) in cancer tissues, circulating autoantibodies to these glycated conjugates of proteins and nucleic acids [7, 8] and overexpression of receptor for AGEs (RAGE) in cancers of the brain, breast, lung, colon, oral squamous cell, ovarian, prostate, pancreatic, lymphoma, and melanoma [9,10,11].
AGEs have been known to cause mutagenesis [12], protein misfolding [13], and loss of protein functions [14, 15] and stimulate proliferation and migration of cancer cells [16,17,18] through a cell surface, membrane bound, pattern recognition receptor, RAGE, which is a member of Immunoglobulin superfamily, and binds to a broad repertoire of ligands besides AGEs. These include β2 integrin/Mac-1, amyloid β-peptide, DAMPs (damage-associated pattern molecules)—high-mobility group box HMGB1/amphoterin and S100 group of proteins/calgranulins [19]. RAGE is expressed in a wide variety of cells, such as smooth muscles, nerve cells, endothelial cells, macrophages, neutrophils, mast cells (immune cells), and cancer cells. RAGE (gene) is located on chromosome 6, in the MHC class III region of the major histocompatibility complex, concerned with immune responses. The perpetuated signalling flux of the RAGE ligand-receptor-transcription factor complex (ligand-RAGE-NF-ķB) has been found to contribute to multiple diseases, such as Alzheimer’s, atherosclerosis, diabetes, obesity, arthritis, PCOS (polycystic ovarian syndrome), certain neuronal, nephrological, and respiratory illnesses, besides cancer [20,21,22,23,24,25,26].
RAGE upon stimulation by its ligands—AGEs, HMGB1, and S100 group of proteins, leads to the activation of several molecular signalling pathways—PI3K/AKT, JAK/STAT, NF-ķB, Ras/MAPK, Rac1/cdc42, p44/p42, p38, and SAP/JNK MAPK and transcription factors, such as NF-ķB, STAT3, HIF-1α, AP-1, and CREB [27,28,29,30]. The widely varied genomic architecture of tumours subsidised by accumulating mutations due to uncontrolled cell division, improper repair machinery, and high oxidative stress confers resistance to the cancer cells from chemotherapy and radiotherapy [31, 32]. The divergent yet interlinked signalling network of neoplasms make curbing the disease difficult with isolated chances for single gene targets unique to individual molecular subtypes of cancer. This current scenario of onco-therapeutics necessitates research focus on specific molecular mechanism or pathway, which has a role in different stages and types of cancer starting from oncogenic transformation of normal tissue flora to survival and metastases of cancer cells [33].
Metabolic perturbation of tumours makes them universally similar, setting aside their individual variability on the basis of any molecular or site-specific characteristics [34]. Distinct molecular mechanisms intertwining the shift from mitochondrial respiration to enhanced glycolytic flux mark the dynamic change from small unchecked growths into aggressive tumours, with receptor for advanced glycation end products (RAGE)-ligand signalling axis involved in the processes leading to the origin and invasion of cancers [35,36,37].
Despite noteworthy contributions to studies on RAGE and its ligands in cancer, the detailed mechanisms involved their clinical and therapeutic implications, as early detection markers and drug targets are yet to be fully elucidated. The ligand-receptor (RAGE- AGEs/HMGB1/S100) interaction determines the degree of metastatic spread, aggressive, and recurrent nature of the disease, besides contributing to oncogenesis and eliciting chemotherapy resistance.
RAGE plays a master regulator of origin, invasion and metastasis of tumours by binding with AGEs, HMGB1, or S100 group of proteins, which are expressed largely during pathological conditions of glycation and inflammation. The receptor-ligand duo serves as the key target for prevention and successful treatment of cancers, irrespective of their site of origin, molecular subtype, and disease stage. It confers selective cytotoxicity to drugs targeting RAGE and its ligands, which when identified could be used in combination with standard conventional chemotherapy for effective control of progression and metastasis of cancers, simultaneously devoid of any adverse effects to normal cells. RAGE is highly expressed in a vast majority of cancers but expressed very low under physiological conditions; hence, the RAGE-ligand duo can be marked as a lead target for emerging novel anti-neoplastic agents.
This review highlights the molecular mechanism underlying RAGE-ligand interaction, mainly AGEs and also HMGB1 and S100 proteins related to glycoxidative stress, inflammatory niche, cancer stem cell (CSC) re-activation, and cancer hallmarks. The implications of early screening for the (s)quad of tissue RAGE, serum AGEs, circulating soluble RAGE, and RAGE gene polymorphisms in cancer patients for assessing their diagnostic and prognostic significance and intervention with inhibitors of the RAGE-ligands are also discussed. In this review, we have accentuated the significant interplay of RAGE-ligand signal network (especially AGEs), linking glycation, inflammation, and the hallmarks of cancer, implying the same for timely diagnosis and effective targeting of neoplasms with RAGE-ligand targeted therapeutic agents.
Glycolysis, Glycation, and Cancers
Abnormally altered cellular respiration upholding glycation reactions and opening the gateway of AGE-RAGE signalling axis, mechanistically critical to the development of malignancies, are discussed.
Cancer Metabolism and Glycation—AGEs
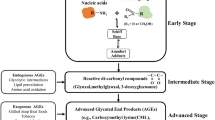
Metabolic dependence of tumours on glycolysis even under adequate oxygen supply (Warburg’s hypothesis) and anaerobic glycolysis under intratumoral hypoxia lead to an upsurge in glucose uptake and turnover of glycolytic intermediates [38, 39]. The subsequent hyperglycemic micromilieu coupled with the generation of reactive oxygen species favours the emergence of glycoxidative adducts called AGEs. The non-enzymatic glycation of proteins, lipids, or nucleic acids by the reaction between carbonyl group of reducing sugars and amino groups of proteins or nucleic acids results in the formation of AGEs [40]. This reaction occurs under the presence of a high abundance of reducing sugars such as glucose, fructose, ribose (ADP-ribose from DNA strand breaks), and intermediates of glycolysis/dicarbonyl derivatives, such as glyceraldehyde, glyoxal, methyl glyoxal (MG), 3-deoxyglucosone (3-dG), and glucose-6-phosphate, which are potent glycating agents. AGEs can be formed from different sources inclusive of Maillard’s reaction, polyol pathway, lipid peroxidation, autoxidation of glucose, high oxidative stress, and glycolysis pathway, almost all of which require hyperglycemic cellular microenvironment [41, 42].
Glycolytic Dependency and Cancer Malignancy—AGEs and RAGE
Glycolytic abundance of cancer cells fuel the glycation and oxidation reactions in a tumour microenvironment, which facilitate cancer progression through the generation of AGEs and their receptor (RAGE)-mediated molecular cues [43]. Both tumours and associated stroma exhibiting Warburg’s glycolytic phenotype depict biologically aggressive nature with poor prognosis, reporting upsurge in GLUT-1 and Ki-67 levels, as revealed in a Korean-based population study [44]. Hormonal-dependent as well as independent tumours, featuring Warburg phenotype and/or high RAGE expression, show highly elevated mitotic and metastatic indices. Studies have shown that AGEs, the by-products of abnormal glucose metabolism, upregulate the oncogenic processes of both hormone-dependent and independent cancers, with its pronounced expression leading to malignant behaviour of cancers via intensified RAGE expression [4, 18]. Highly progressive and metastatic cancers exhibit proportionately amplified RAGE expression when compared with the less aggressive and primary cancers [45,46,47]. This implies the concrete involvement of AGE-RAGE signalling axis in most aggressive cancers presenting accelerated glycolytic rate. The AGE-RAGE duo links the glycolytic abundance of cancers with their metastatic propulsion to secondary sites.
Implications of Protein and DNA-AGEs in Cancer
RAGE-Independent Effects of AGEs
In addition to RAGE, its ligands are found to be over expressed in most types of cancer [9, 10]. AGEs, besides binding with the receptor, exert adverse events at cellular and molecular level (mentioned in Table 1), by directly interacting with bio macromolecules and causing structural perturbations, loss of activity, unfolding, degradation of proteins, induced mutations, single strand breaks, and unwinding of DNA.
The glycation of proteins and DNA is widely seen in several cancers besides diabetes, evidenced by the presence of various AGE modified proteins in invasive ductal carcinoma (IDC) of the breast and CEdG (MG or 3-dG modified DNA) in sera and tissues of different cancers [6, 8]. Identified AGE-modified proteins in cancer tissues include prohibitin, glycerol-3-phosphate dehydrogenase and lactate dehydrogenase, annexin II, MG-modified histone proteins, serotransferrin, and fibrinogen with possible implications in cancer progression [48]. The AGE-modified biomolecules have been found to be immunogenic and genotoxic, reported by the existence of autoantibodies against, such glycated adducts as MG modified histone and MG or 3-dG modified DNA in cancer patients [7, 53].
Histone proteins are involved in packing DNA into chromatin and the regulation of gene expression. Disturbances in the structure and functions of H2A through glycation by methyl glyoxal or (ADP) ribose can cause loss of chromatin integrity and impaired epigenetic modulations leading to genomic instability. H1 and H2B upon glycation have displayed alterations in chromatin structure and genomic instability developing into undesirable effects leading to various diseases. In addition, H2A is known to be involved in host immune responses (anti-microbial peptide),and its glycation is related with eliciting autoimmune responses in cancer [52].
Non-enzymatic glycation of haemoglobin alters its structure and function, increasing its affinity for oxygen and thereby reducing the delivery of oxygen to tissues. This glycated haemoglobin could diminish oxygen supply to tumour tissues, resulting in hypoxia [50]. Hypoxia enables cancer cells to overcome nutritive deprivation by up regulated glycolysis to escape the hostile environment, by altering the genome and proteome of neoplastic cells. Hence, hypoxia favours hyperglycemic milieu for AGEs production and the modification of molecular milieu including vascular components by AGEs in turn result in hypoxia [54].
Altogether, glycated proteins or AGEs by themselves have the potential to support and augment cancer related cellular and molecular events, besides perpetuation of cancer development via RAGE binding.
RAGE-Dependent Effects of AGEs
RAGE is comprised of three extracellular domains, V, C1, and C2, a trans-membrane helix, and a short cytoplasmic tail. Receptor-mediated effects of AGEs involve interaction with RAGE V domain [55]. Major AGEs found in tissues and plasma (in cancer and physiological conditions) that are also ligands of RAGE include CML, CEL, and argpyrimidine. This interaction is concomitant with chronic inflammation and cancer, besides diabetic complications and Alzheimer’s disease.
AGE modifications observed in cancer tissues include [48]:
-
Pyrraline
-
Imidazolone A & B
-
Argpyrimidine (a MG modification)
-
Fructosylysine
-
Methyl glyoxal lysine dimer
-
Nε-(carboxyethyl)lysine (CEL)
-
Nε-(carboxymethyl)lysine (CML)
The preferential AGE modifications identified in cancer tissues include CEdG-DNA adducts, argpyrimidine, and CML-modified protein adducts. These AGE adducts have been reported both in tumours and adjacent normal tissues of the breast, colon, and other cancers, indicative of their role in the onset as well as progression of cancers [6]. AGE-RAGE signalling of stromal components has also been implied in cancer growth.
Argpyrimidine modification of HSP-27 (heat shock protein) plays a role in suppressing cyt-c-induced caspase activation, and its inhibition was found to sensitise cancer cells to drug-induced apoptosis. Nuclear, cytoplasmic, and extracellular argpyrimidine modifications have been observed in tumours. Intracellular AGEs cause DNA mutagenesis and protein dysfunctions resulting in the abnormal genome and proteome, eliciting disruption of growth checkpoints for cancer initiation and survival [56], unlike extracellular AGEs acting upon tumour initiation and spread via RAGE binding [16]. This is further established by the glycoxidative lesions identified in lymphocyte DNA from cancer patients [7] and augmented expression of AGEs, RAGE, and NF-ķB predominantly in tumour tissues of invasive carcinoma than the corresponding control [48, 57].
Glycated and diabetic HDL have been observed to promote cancer survival, growth, and invasion in breast cancer [49]. Glucose, glyceraldehyde, and methyl glyoxal AGEs positively attribute to proliferation and migration of oral, prostate, colorectal, breast, melanoma, and lung cancers [4, 16, 57,58,59]. They are facilitated via RAGE mediated upsurge in the expression of different genes involved in cancer promotion, such as NOX-2, NF-ķB, SP-1, MMP-2 and -9, Bcl-xl; phosphorylation of ERK 1/2, p38 MAPK, STAT-3, and p70S6K1; down regulation of Nrf-2 and Bcl-2; p53 expression; and stimulation of PI3K/Akt pathway.
All these factors point towards the association of AGEs with the process of malignant transformation via both RAGE-dependent and -independent mechanisms.
RAGE/Ligands—Glycation, Inflammation, and Hallmarks of Oncogenesis
Formation of AGEs occur not only under conditions of hyperglycemia and enhanced glycolysis (in cancer) but also with ageing, inflammation and oxidative stress [10, 48]. Therefore, besides hyperglycemic conditions, chronic states of inflammation and oxidative stress also predispose to cancer initiation as in ageing, obesity, and diabetes, leading to the generation of AGEs and higher expression of RAGE (detailed in Fig. 1). These further provoke the oncogenic transformation of precancerous lesions and normal tissues [60]. Different AGEs of glucose, glyceraldehyde, and methyl glyoxal promote cancer growth. Mainly, methyl glyoxal AGEs with concomitant oxidative/carbonyl stress contribute to the propagation of various cancers. MG-AGEs are known to worsen the pre-existing neoplastic lesions, by accelerated inflammatory, oxidative stress markers, and acting as a potent glycating agent [61]. Intracellular hyperglycemia-induced ROS production by mitochondrial electron transport chain and intratumoral hypoxia accelerate the production and release of endogenous RAGE ligands S100A4, S100A8, S100A12, and HMGB1 (DAMPs) besides AGEs. Expression of the DAMPs has also been implicated in inflammation and cancers [62]. Sustained inflammation and oxidative stress stimulate the overexpression of RAGE, leading to the production of pro-inflammatory cytokines [63].
Sequential cellular and molecular events leading to oncogenesis initiated by the setting of persistent glycoxidative and inflammatory stress, attributed by the generation of RAGE ligands—AGEs, S100, and HMGB1 and subsequent activation of RAGE, favouring the derivation of hallmarks of a tumorigenic milieu, which include metabolic reprogramming, sustained growth signals, sustained inflammation, promotion of angiogenesis, genomic instability, evasion of tumour suppression, and telomere elongation, ultimately subsidising oncogenic transformation and cancer promotion
The chronic inflammatory and glycoxidative cues nourished by DAMPs and AGEs respectively enrich the tissue micro milieu for the establishment of a pre-malignant niche. Multitude of signal network spinning glycation and inflammation with initiation of cancers via RAGE-ligand-assisted development of oncogenic hallmarks are discussed.
Sustained Inflammation and Oncogenic Transformation—AGEs
Under persistent conditions of hyperglycemia (as glycation in diabetes) and/or inflammation (as in obesity), leading to oxidative stress (as in ageing), high glucose and methyl glyoxal AGEs stimulate the binding of NF-ķB (Nuclear factor-ķB) to RAGE promoter and AP-1(Activator protein-1) to S100/HMGB1 promoters [62], inducing the expression of RAGE and S100/HMGB1 molecules, respectively. AGEs augment RAGE mRNA as well as protein expression levels. Extracellular AGEs bind with RAGE and promote downstream enhancement of NADPH oxidase enzyme 2 (NOX2), ROS production, and NF-ķB, which in turn stimulates RAGE expression by binding to its promoter [62, 64]. RAGE is a strong inducer of NF-ķB activation, by de novo synthesis of RelA messenger RNA, maintaining a perpetual derivation of transcriptionally active NF-ķB, linking inflammation to the development of cancers. Consequently, NF-ķB stimulation by RAGE-ligand engagement drives inflammation and tumorigenesis [65]. AGE-RAGE binding via NF-ķB upsurges the transcriptional activation of different genes involved in inflammation including cell adhesion molecules like E-selectin, ICAM-1, and VCAM-1 [63]. Incessant AGE accumulation and RAGE activation lead to the secretion of pro-inflammatory cytokines (IL-6, 8; TNF-α) and adhesion molecules. RAGE also serves as an adhesion receptor for β2 integrin Mac-1 secreted from immune cells, facilitating the engagement of leukocytes and promoting inflammation [66]. RAGE interaction with S100 proteins (S100B and S100A12) also induces VCAM-1 expression.
Hence, once initiated, AGE/RAGE/NOX-2/NF-ķB is a self-sustained signalling axis, promoting inflammation and cancers [67]. All the four components of this axis have been reported to be expressed in high levels in cancer tissues in comparison with control [48].
Metabolic Reprogramming and Sustained Growth Signals—AGEs
RAGE acting as an oncogene under stimulation by its ligands couples with the inflammatory mediators RAS and NF-ķB to establish the metabolic paradigm of tumour and adjoining microenvironment [68]. Sustained accumulation of AGEs activate RAGE/ RAS/NF-ķB signalling cascade and thereby evoke unrestrained growth signals by up regulation of HIF-1α (hypoxia inducible factor-1α) [30], besides induction of HIF-1α by hypoxia prevalent in more than 90% of tumours. HIF-1α enhances the expression of proteins concerned with glucose transporters (GLUT-1), glycolytic enzymes, and growth factors (TGF, IGF, EGF, VEGF). This shifts the cellular energy derivation from mitochondrial respiration to glycolysis unlimitedly promoted. Metabolic reprogramming of cancers ensues, thus qualifying the ultimate requisite for unhindered mounting up of oncogenic processes [69]. Hypoxia stimulates RAGE expression via HIF-1α mediated rise in expression of its ligands, S100A8 and S100A9 [70]. The greater the dependency of cancer and stromal cells on glycolysis, the higher are the generation of AGEs, lactate, and expression of associated genes like Ki-67 (proliferation index), HIF-1α, GLUT-1, and MMPs in correlation with the malignant grade and biologically aggressive nature of tumours [44]. The higher the glycolytic turnover, the lesser the dependency on mitochondrial respiration and the greater the build-up of AGEs and RAGE signalling, furthermore stimulating HIF-1α and sustaining the feedback loop towards complete metabolic shift favouring oncogenesis.
Promoting Angiogenesis
NADPH pathway is involved in oxidative stress pertaining to RAGE stimulation [71]. NADPH oxidases (NOX) belong to membrane bound respiratory burst oxidase of leukocytes. Enhancement of NOX by RAGE, inflammation, hypoxia, and growth factors—VEGF and angiopoietin promote ROS-driven autophosphorylation of VEGFR2, stimulation of redox signalling pathways, and angiogenic transcription factors, in the initial as well as late stages of cancers. Upregulated NOX is associated with cellular and DNA damage via ROS, leading to the development of pre-malignant niche concomitant with the onset and progression of cancers. AGEs cause an upsurge in NADPH oxidase levels and oxidative stress via RAGE overexpression leading to promotion of angiogenesis and hence tumorigenesis [66, 72]. Blockade of RAGE inhibits angiogenesis of cancers by impeding the expression of VEGF and SP1 protein (detailed in section “Promotion of Tumour Vasculature and Metastasis”) [73].
Sustained Inflammation, Telomere Elongation, and Genomic Instability—AGEs
Telomerase is a reverse transcriptase enzyme, responsible for the lengthening of telomeres, expressed in high levels in most cancer cells [74]. The limited replicative potential of normal somatic cells caused by telomere shortening is reverted back by NF-ķB and PARP (poly-ADP-ribose polymerase). NF-ķBp65 interaction stimulates nuclear translocation of hTERT and transcriptional upregulation of telomerase levels through c-myc, which in turn induces NF-ķB-dependent gene expression, generating a feed forward loop leading to the co-existence of chronic inflammation and sustained telomerase activity seen in tumours. The upregulation of telomerase activity facilitates the re-entry of senescent cells into cell cycle, a vital step in the early stages of carcinogenesis [75]. Recently non-canonical functions of telomerase have been identified for their role in hallmarks of cancer with potential implications in oncotherapeutics. These include favouring cell proliferation, evading apoptosis, metabolic reprogramming, invasion, and drug resistance [76]. Further, translocation of hTERT between nucleus and mitochondria is regarded an adverse prognostic factor for neoplasms [77]. Upon AGE-RAGE interaction, there is constant induction of NF-ķB and telomerase levels by a bidirectional activated channel, favouring a niche for the generation of hallmarks of cancer [78, 79].
Activated PARP upon DNA strand breaks induced by AGEs and ROS generation (in intracellular hyperglycemia) elicits alternative lengthening of telomeres, which aids in the replicative immortality required for tumorigenesis. PARP 5a, an isoform of PARP, facilitates telomere elongation in the context of telomerase inhibition, consistent with its high expression in cancers (mainly breast and gastric). PARP5a inhibition leads to telomere shortening without affecting telomerase activity [80]. Additionally, PARP activates protein kinase C (PKC) isoforms, together with Polyol and hexosamine pathways, all of which have implications in the initiation and propagation of cancers [62].
Thus, NF-ķB by enhancing telomerase levels and PARP by directly acting on telomeres rather than telomerase both couple to bring about immortal replicative potential enabling tumorigenesis. PARP also enhances transcription and activity of NF-ķBp65, increases the levels of AGEs in the cells, further perpetuating inflammation and glycation. Glycation of DNA and histone proteins by AGEs inflict chromatin and genomic damage, further inducing mutations characteristic of tumorigenesis (detailed in section “RAGE-Independent Effects of AGEs”).
Sustained Inflammation and Cancer Initiation—DAMPs
Several inflammatory pathways are activated upon ROS induction following RAGE-ligand interaction. Besides stimulated expression by AGE driven AP-1 binding, DAMPs like HMGB1and S100 group of proteins are secreted from immune cells under inflammation, tissue injury, necrosis, and hypoxia [81]. Following RAGE interaction, they instigate downstream molecular events contributing to cancer growth. Together with AGEs, S100 and HMGB1 also stimulate RAGE expression by inducing SP-1 binding to RAGE promoter. HMGB1 is a member of non-histone, chromatin-associated high-mobility group box of proteins, expressed in a vast majority of cancer cells and secreted constantly by necrotic cancer cells, creating a sustained inflammatory niche, concomitant tumorigenesis and malignant transformation [67]. Activated PARP is known to facilitate HMGB1 translocation from nucleus to cytoplasm for release on necrotic death [82]. HMGB1 serves intracellularly as a DNA chaperone (physiological function) [83] and extracellularly as a cytokine and damage-associated pattern molecule (pathological function), indicative of its potent role in promoting malignancies, only upon cellular release [84]. Majority of cancer cell death cause HMGB1 release, thus intensifying RAGE signalling alongside AGEs, making the tumour phenotype aggressive, therapy resistant and eliciting tumour resurgence [85].
S100 family of small molecular weight calcium-binding proteins is prevalent in a wide variety of inflammatory diseases and upregulated in many cancers. They are expressed by neutrophils, macrophages, lymphocytes, and dendritic cells. S100 proteins that bind RAGE with reported presence in cancer tissues include S100A4, S100A6, S100A7, S100A8/9, S100A11, S100B, and S100P. S100A8/9, a key pro-inflammatory mediator in acute and chronic inflammation, depicts elevated levels in chemical induced carcinogenesis via RAGE induction, eliciting a positive regulatory loop of chronic inflammatory setting essential for tumour promotion [86,87,88].
Blockade of RAGE-HMGB1/S100 interaction has been found to reduce inflammation and cancer [89, 90]. RAGE knockdown has displayed attenuated levels of pro-inflammatory mediators (mainly S100A8/9 and macrophage inflammatory proteins) and infiltrating immune cells, resulting in impaired tumour formation in animal models.
Taken together, apart from DAMPs, AGEs adopt various RAGE dependent and independent mechanisms to create a link between an array of hallmarks of oncogenesis such as dysregulated cellular metabolism, persistent growth signals, evasion of tumour suppression, sustained inflammation, and telomere elongation, ultimately resulting in a well-established tumour initiating microenvironment.
RAGE—Ligands and Hallmarks of Cancer Progression
Following the crosstalk between various oncogenic hallmarks, the perpetuated RAGE-ligand signalling further leads to the evolution of several downstream signalling pathways, ensuing in the emergence of well-refined and interlinked network of absolute hallmarks of cancer progression.
Hypoxia of tumour niche and elevated glycolysis, followed by hyperglycemia and glycation, sustain the generation of AGEs and oxidative stress [48], augmenting RAGE signalling pathway to elicit development and propagation of cancers. Both cancer cells and the stromal components (tumour associated fibroblasts, leukocytes, endothelial, and smooth muscle cells) secrete RAGE ligands (HMGB1 and S100) and express RAGE in an autocrine and paracrine fashion, contributing further to the sequential molecular events (explained in Fig. 2) responsible for cellular transformation, cancer proliferation, invasion, metastasis, and chemo-resistance [10].
Cascade of signalling events triggered by RAGE-ligand interaction, leading to cancer proliferation, survival, autophagy, cell motility, invasion, CSC renewal, chemo-resistance, and different hallmarks of cancer inclusive of immune suppression, evasion of apoptosis, promotion of epithelial mesenchymal transition (EMT), and metastasis, via activation of various molecular pathways and induction of transcription factors
RAGE establishes sustained cellular activation by enabling the generation of its own extracellular ligands (Fig. 2). The cytosolic domain of RAGE lacks intrinsic tyrosine kinase activity or any known motifs engaged in downstream effector signalling. RAGE binds with diaphanous-1 (Dia-1), a member of formin protein family, which serves to reorganise actin cytoskeleton, regulate cell motility, and elicit signalling via transcription factors. Both RAGE and Dia-1 are implicated in the regulation of inflammatory, vascular, and transformed cell migration [91].
Sustained Growth Signals, Evasion of Apoptosis, and Tumour Suppression
PI3K/AKT Signalling Pathway—AGEs
The PI3K/AKT signalling pathway controls events concerned with cell cycle and evasion of apoptosis by inhibiting pro-apoptotic proteins, FasL, Bim, Bad, and BAX and activating anti-apoptotic genes, Bcl-2, and NF-ķB [92]. Initiation of RAGE-ligand splicing facilitates cell cycle progression through G1-S phase via phosphorylation and ensuing activation of PI3K/AKT signal molecules. Studies show cell cycle arrest in G1 phase, decreased expression of NF-ķBp65, and proliferation on RAGE knockdown in cancer [93].
Normally, Rb (tumour suppressor) binding E2F (family of cell cycle transcription factors) suppresses gene transcription related to G1-S phase transition and thereby cell proliferation. But AGE-RAGE interaction activates PI3K/AKT axis, promoting G1-S progression and hence proliferation, by phosphorylation and subsequent decrease of Rb family of proteins via cyclin D-dependent kinases (cdks 4/6). Blockade of PI3K/AKT cascade caused disruption of AGEs induced cancer proliferation, indicating it as a prominent pathway of action of AGEs [16].
ERK1/2 Signalling Pathway-AGEs
Sustained upregulation of ERK1/2 MAP kinase pathway is required for cell proliferation and cell cycle progression [94]. ERK1/2 signalling is mainly linked with cyclin D1 promotion and G1-S phase progression, via induction of positive regulators of cell cycle [95]. Ras/RAF/MEK/ERK1/2 signalling axis with a significant role in cancer progression is activated downstream RAGE-ligand interlocking [96]. RAGE knockdown studies have revealed down regulated expression of proliferation markers PCNA and Cyclin D1, causing proliferative inhibition [93]. Cyclin D1 upregulation contributes to malignant transformation consistent with the malignant phenotype of RAGE over expressing tumours.
Substrate phosphorylation of ERK1/2 results in pro-proliferative, angiogenic, and invasive effects. HMGB1 [89, 97] and S100 proteins [86, 98] prompt RAGE mediated cancer proliferation and invasion mainly through MAPK and NF-ķB pathways. Blockade of RAGE-HMGB1 interaction diminished tumour growth and metastases in implanted as well as spontaneously developed tumours in mice, via suppression of upregulated ERK1/2, p44/p42, p38, SAP/JNK MAP kinases, and attenuation of MMP expression. Hence, HMGB1 stimulates cancer progression through RAGE mediated MAPK signalling [89]. Furthermore, RAGE-HMGB1 interplay aids autophagy by activating Beclin-1 via ERK-mediated phosphorylation of Bcl-2 [97].
AGEs via RAGE mediated increase in intracellular ROS augment EGFR and ERK1/2 protein phosphorylation inducing mitogenesis [96]. EGFR is entailed in activating tumour and adjacent stroma creating a favourable niche for cancer invasion and metastasis. Studies have shown AGE induced p44/42 MAP kinase activation via RAGE, affecting inflammatory and proliferative pathways [99]. Upregulated RAGE was found to elicit MEK-EMT signalling in vitro and lung metastasis in vivo, independent of tumour growth [100]. Using RAGE knockdown models in vitro and in vivo, significant reduction in cancer growth, angiogenesis, metastasis, and inflammatory cell recruitment with downregulated MAPK signalling was observed [93, 101, 102].
JAK/STAT pathway—AGEs
AGEs augment the proliferation and invasion of several cancers, including breast and colorectal, via activation of RAGE and several transcription factors including STAT-3 [4, 61]. Pim-1 and Cyclin D1, triggered by JAK/STAT signalling, are proliferative signals for cancer cells, with Pim-1 facilitating protection from apoptosis [103].
Evasion of Apoptosis and Tumour Suppression
NF-ķB upon RAGE stimulation promotes anti-apoptotic signals besides activating pro-inflammatory genes [104]. Repression of PTEN expression is elicited by NF-ķB and IL-1β signalling [105]. PTEN inhibition results in dysregulated cellular metabolism and has been implied in cancers. In the framework of metabolism and cancer, PTEN, IL-1β, and RAGE are interlinked. PTEN is a tumour suppressor inhibiting PI3K/AKT pathway entailed in neoplastic progression. Suppression of PTEN occurs downstream AGE-RAGE interlocking [104], one of the potential mechanisms of evading tumour suppression and eliciting oncogenic molecular processes via PI3K/AKT signal progression.
p53 is a target of S100 proteins—S100B, S100A2, and S100A4. These proteins transcriptionally modulate p53 and inhibit its tumour suppressor functions [106, 107], thus evading apoptosis and contributing to cancer development [108, 109]. Detachment of p53 from S100B has been found to initiate apoptosis and abrogate cancer cell migration in cancers by hindering MMP-2. RAGE exhibits resistance to programmed cell death via p53-dependent mitochondrial pathway. RAGE checks apoptosis and facilitates enhanced autophagy and tumour survival via increased Beclin-1 autophagosome formation [85]. Under necrotic death, HMGB1 is released from dying cells, eliciting tumour-associated inflammation, RAGE signalling, and hence resist oncotherapy causing tumour relapse. Altogether, RAGE is an indispensable factor contributing to a pool of intra-/extracellular and molecular events essential for the growth and propagation of tumours via promotion of persistent proliferative signals, elusion of apoptosis, and inhibition of tumour suppressors.
Promotion of Tumour Vasculature and Metastasis
RAGE drives cell migration through Rac-1/cdc-42 via interaction with its intracellular binding partner Diaphanous1 [91]. Rac1/cdc42 belongs to Rho GTPases, a family of small GTP-binding proteins that regulate actin cytoskeleton dynamics and play a vital role in cell motility and its adaptability to the tumour niche [91]. Mainly concerned with migration and proliferation kinetics, these proteins have major functions in invasive and aggressive behaviour of cancers. S100A4 combines with VEGF through RAGE and supports angiogenesis [110].
RAGE shRNA transfection studies showed RAGE directed angiogenesis and invasion of cancers and blockade of the receptor resulting in diminished expression of VEGF and Sp-1, both in vitro and in vivo [73]. Elevated expression of RAGE, Sp-1, MMP-2, and presence of surplus glucose AGEs have been identified in the sera and tumour tissues of colorectal cancer patients from a Chinese population. Addition of external AGEs revealed proportionate surge in RAGE, Sp-1, MMP-2 levels, and phosphorylation of ERK1/2 facilitating migration and invasion in vitro, similar to in vivo conditions. RAGE expression was found to be correlated with proliferative index, metastatic grade, and TNM stage in several cancers. RAGE interference mitigated proliferation and microvessel formation in endometrial cancer in vitro; RAGE levels also positively correlated with the vascular density of human endometrial cancer samples, indicative of its role in angiogenesis besides invasion and metastasis [111].
Bone metastasis of breast tumours with high RAGE expression is attributed to the osteolytic lesions induced by RAGE at sites of metastasis. Studies show RAGE-mediated tumour progression and macrophage recruitment to tumour niche by AGEs [4] and S100A7 [102] in MDA-MB-231 cancer cells and transgenic mice models, respectively. RAGE and methyl glyoxal AGEs regulate the proliferation, chemo-taxis, and migration of different cancers mainly TNBCs, known primarily for high metastatic and poor prognostic nature [93]. Moreover, high RAGE expression was observed in more than 50–60% of IDC of the breast, in correlation with the elevated expression of AGEs, AGE-modified proteins, NF-ķB, and NOX [48]. Different studies imply the interplay of RAGE-ligand signalling network in eliciting the angiogenesis and metastasis of different cancers.
Invasion and Tumour-Associated Inflammation
Hypoxia/HIF-1α driven increase in S100A8/A9 has been observed in prostate cancer in vitro, promoting invasion via RAGE interaction, NF-ķB activation, and subsequent upsurge in MMP (2 and 9) levels [88]. Thus, NF-ķB elicits inflammation as well as invasion associated expression factors. Real-time augmented expression of these S100 proteins is observed in several cancers and tumour-infiltrating immune cells. Induction of metastasis proportionate with RAGE-HMGB1 levels has been observed in head and neck squamous cell carcinomas, with over expression of the duo in tumour tissues [112]. RAGE RNAi studies in cancer cells displayed proliferative inhibition and Caspase-3 and -8-mediated apoptotic induction; in mice cancer models, they exhibited downregulated DR4 and DR5 expression with attenuation of RAGE- HMGB1 facilitated tumour growth [101].
Altogether, of the different molecular pathways initiated downstream RAGE-ligand interaction, ERK and AKT signalling axes appear to be the major pathway of action for RAGE ligands with concomitant implications in several cancers. NF-ķB, downstream effector molecule of RAGE, promotes pro-inflammatory as well as anti-apoptotic genes, linking sustained inflammation and cancer (detailed in “Sustained Inflammation and Oncogenic Transformation—AGEs,” “Sustained Inflammation, Telomere Elongation, and Genomic Instability—AGEs,” and “Sustained Inflammation and Cancer Initiation—DAMPs”). RAGE upregulates COX-2, an active downstream target of NF-ķB and ERK/AKT pathways, which triggers oncogenesis, angiogenesis, and metastasis via PGE2 production. This signifies the interface of RAGE-ERK/AKT-NF-ķB axis in inflammation-associated neoplastic progression.
Evasion of Immune Suppression
Augmented expression of RAGE is linked with propagation of cancers via chemo resistance and evasion of apoptosis (described in “Evasion of Apoptosis and tumour Suppression”) [85]. Hence non-responsiveness to chemotherapy, radiotherapy, and immunotherapy accompanied by poor prognosis is seen in different cancers. This is due to ROS generation and HMGB1 production by stressed or necrotic tumour cells and immune cells, resulting in HMGB1-RAGE-ERK facilitated autophagy (via Beclin-1, LC-3) [97] and suppression of the immune system (via CTLA4) [104], causing therapy resistance and survival of cancer cells.
T regulatory cells exhibit central role in immune suppression in injury, sepsis, and cancers via upregulated expression of CTLA-4, an immune checkpoint activated by HMGB1-RAGE binding on the surface of T regulatory cells. Blockade of immune checkpoint by anti-CTLA4 antibody is an important element of contemporary immunotherapy-based drug development research, shown to display long-lasting therapeutic responses among patients with different cancers [113].
Increased circulatory myeloid suppressor cells (MDSCs) in cancers have been linked with enhanced cancer development and metastasis by means of promoting immune evasion. Circulating levels of MDSCs in prostate cancers are correlated with PSA levels and metastatic grade. Recruitment and promotion of MDSCs in cancers are induced by S100A8/9 and AGEs binding RAGE and activating NF-ķB dependent mechanistics [104, 114]. Recent prostate cancer mouse model study published in Nature [113] has established the efficacy of combinatorial treatment of anti-CTLA4 antibody and MDSC targeted therapy against both primary and metastatic cancers, emphasising the notable efficacy of targeted therapy in combination with traditional oncotherapy.
RAGE-ligand interaction, the upstream regulator of both CTLA-4 and MDSCs, plays a significant role in their augmentation facilitating immune suppression. The duo of receptor-ligand serves as potential target for developing novel oncotherapeutics, even based on immunotherapy, effective in different stages, hallmarks and types of cancer.
Osteopontin, RAGE/Ligands, and Cancer
In addition to RAGE, AGEs are known to up regulate the expression of osteopontin [115, 116], which binds with cell membrane bound integrin and CD44 and sets up a signalling cascade contributing to inflammation and tumour development [117, 118]. Osteopontin (OPN), a vital protein component of extracellular matrix, has important physiological functions like the regulation of immune response, bone metabolism, and pathological functions like proliferation, angiogenesis, and invasion of cancers [119, 120]. OPN transactivates normal mammary fibroblasts to tumour promoting fibroblasts via TGF-β1, thereby aiding breast cancer progression and metastasis [118, 121]. Augmentation of OPN expression is seen in patients with TNBC [122, 123], a biologically aggressive tumour with Warburg’s glycolytic phenotype with high RAGE expression and also in HER2 over expressing breast cancers [124]. Both RAGE and OPN expression levels are elevated proportionately with metastatic grade, concomitant with poor prognosis [102, 125, 126]. AGEs increase both RAGE and OPN expression levels [116], and the rise in OPN is neutralised by anti-RAGE antibody, implicating the involvement of RAGE in AGE driven OPN expression [115]. HMGB1/RAGE/ OPN/EGR-1 pathway has been implicated in inflammation and angiogenesis of proliferative vitreo-retinal disorders [127] (the modulation of OPN by AGEs and HMGB1 in cancer tissues still needs to be evaluated).
OPN induces nuclear HMGB1 acetylation via activated NOX and abrogated HDAC expression and facilitates its translocation to cytoplasm enabling collagen expression. HMGB1 promotes collagen biosynthesis via RAGE/PI3K/AKT pathway [128, 129]. Though proteosomal degradation of extracellular matrix is required for cellular invasion in cancers, recent evidence reveals the vital role of type-I collagen in paving way for cell migration during invasion [130]. OPN also causes an upsurge in HMGB1 expression and release from tumour and non-tumour cells. OPN prompts HIF-1α and VEGF through PKC/PI3K/Akt pathway [131,132,133] and stimulates breast cancer growth through p70S6k dependent ICAM-1 expression by MEK/ERK pathway [134] and activation of JAK/STAT signalling [135]. OPN via ERK1/2 and p38 MAPK facilitates AP-1-mediated enhanced COX-2 levels and hence PGE-2, contributing to endothelial cell motility, migration, and cancer growth [136, 137]. Hahnel and colleagues have described that osteopontin knockdown elicits diminished survival, enhanced apoptosis, and improved radio sensitisation of MDA-MB-231 cancer cells [138].

Both AGEs and HMGB1 might serve to augment osteopontin via RAGE dependent cell signalling in cancer tissues and adjacent non-tumorous micro milieu. In addition, several genes stimulated downstream RAGE and in cancer are also regulated by osteopontin and vice versa, all of which require further in-depth studies into the molecular mechanisms concerned.
The comprehensive role of RAGE-ligand signalling network in deriving the absolute hallmarks of cancer progression and the intricately woven multiple molecular signal cues forming the framework of progressive cancers have been discussed in detail.
RAGE/Ligands—Cancer Stem Cells and Hallmarks of Metastatic Cancers
CSCs with the pluripotency to generate cancer cells at any period of time remain in small groups, which can be easily missed by regular oncotherapy regimen. They are said to be responsible for the genesis, metastasis, and resurgence of cancers, even many years after treatment [139]. Normal cells expressing CSC markers are associated with cancer risk [140]. Though few in numbers, the self-replicative potential of CSC is hard but equally important to be targeted with therapeutics for successful treatment of cancers, prevention of metastasis, and recurrence. Hence, it is crucial to understand the interplay between the malignant/metastatic tumour niche, RAGE-ligand signal axis, and stem cell pathways in cancers. All these molecular interactions emerging as the complete hallmarks of cancer from its initiation to invasion are also discussed.
Tumour Micromilieu in Metastasis
Paget’s seed and soil theory emphasises the vital role of micro milieu in providing a favourable environment for cancer cells in distant organs, eliciting metastasis [141]. The non-tumorous niche composed of stromal cells, cancer-associated fibroblasts, and tumour-associated macrophages contributes major part to the promotion of metastasis and tumour relapse [142]. Besides causing cancer cell death, the chemotherapeutic drugs possibly evoke differential genetic and functional alterations in normal stromal cells or tumour micromilieu, which could potentially determine the propensity of cancer recurrence post chemotherapy. HMGB1 released from necrotic cells and oxidative stress generated in tumour milieu following oncotherapy (section “Evasion of Immune Suppression”) all contribute towards chemo resistance, cancer cell survival and recurrence [104]. Cancer associated fibroblasts (stromal cells) are also reported to express HMGB1, S100A4 and AGEs, which signifies the role of stromal cells in activating RAGE in cancer tissues. This underlines the use of AGE/HMGB1/S100 inhibitors following conventional cancer treatment in preventing therapy resistance, metastasis, and recurrence of cancers.
Cancer Stem Cells and EMT
Abrogation of CSC has been recommended as an essential component of cancer therapeutics, owing to their dynamic role in the survival and metastasis of cancers [139]. Wnt/β-catenin, Notch, and hedgehog pathways are involved in the differentiation and regeneration of normal stem cells; their dysregulation drives tumour cells to metastasize to distant sites via epithelial-mesenchymal transition (EMT) [143, 144]. Interplay between different stem cell signalling pathways also exists for EMT induction in cancers. AGE-RAGE interaction has regulatory role in the Wnt/β-catenin signalling pathway [145], and S100A4 also is related to β-catenin signalling in facilitating metastasis. Studies demonstrate repression of S100 proteins by the inhibitors of Wnt/β-catenin pathway, calcimycin, and sulindac [146, 147]. Activation of β-catenin via PI3K/Akt and Wnt downstream RAGE signalling facilitates EMT required for cancer spread [148, 149]. AGE-RAGE interlocking enhances TGF-β biosynthesis leading to increased MMP activity [150, 151]. RAGE, HMGB1, and S100 group of proteins also upregulate TGF-β expression and signalling, favouring EMT, a key triggering factor in the process of oncogenesis and metastasis [152, 153]. Both TGF-β and β-catenin augment vimentin and attenuate E-cadherin levels, enabling the adaptation of mesenchymal phenotype crucial for the migration of cancer cells. Protein kinase C activated upon RAGE stimulation evokes cancer stemness, EMT, metastasis, evasion of apoptosis, cancer initiation, and survival. PKC is a major therapeutic target for CSC and facilitates metastasis via CXCR4, MMPs, and adhesion molecules [154,155,156].
Instigation of RAGE stimulates CXCR4 expression in mesenchymal stem cells paving way for tumour progression [157]. OPN upregulates TGF- β, CXCR4, and MMP-2 levels [117, 158]. Both CXCR4 and OPN are hypoxia inducible genes activated following RAGE stimulus, which favour homing and adherence of cancer cells to bones and hence, bone metastasis of breast, and other cancers [130]. Apart from RAGE and its ligands, hedgehog ligands secreted by cancer cells also augment OPN, which enhances osteoclast differentiation and activation by elevating cathepsin K and MMP-9 thereby promoting breast cancer bone metastasis. Hence, hedgehog signalling activated upon RAGE-ligand interlocking serves to uphold osteoclast activity and bone metastasis [159]. Inflammatory tumour niche or stromal fibroblasts trigger Snail-1-dependent EMT, whereas hypoxic tumour cells provoke Twist-mediated EMT [160]. Instigation of hypoxia and HIF-1 regulate NOTCH signalling pathway; promote EMT, cell motility, and (bone) metastasis by eliciting mesenchymal markers and suppressing E-cadherin via upregulation of SNAIL, SLUG, TWIST, AXL, ZEB1, CD47, and TAZ, enabling the renewal of CSC-like phenotype.
HMGB1 signals via CXCR4 besides RAGE and Toll-like receptor (TLR) [161, 162]. HMGB1-RAGE-NF-ķB axis actively participates in EMT and metastatic spread, via MAPK-mediated promotion of stem cell proliferation and differentiation. HMGB1 couples with TLR and activates STAT-3 signals, instigating the self-renewal of breast CSC and aiding metastasis [163, 164]. A strong link has been established between PRR and DAMPs, in prompting cancer stemness, epithelial plasticity, and autophagy, vital factors promoting the aggressive behaviour of cancers. Cancer stemness of the cells is responsible for drug resistance, metastasis, and recurrence of cancers [165]. RAGE-HMGB1/S100 signalling axis represents a characteristic prototype of PRR-DAMP interaction, providing avenue for effective targeting of CSC in oncotherapeutics.
Cancer Stem Cells, Diabetes, and Cancer
RAGE (and its ligands) expressing cells are concomitant with the acquisition of CSC-like characteristics, responsible for eliciting tumorigenesis [153]. Hence, a pro-tumorigenic niche capable of triggering RAGE via both paracrine and autocrine secretion of its ligands HMGB1, S100, and AGEs can be entailed in the development of CSC, which in turn instigate cancer, especially in diabetics, possessing pre-existing provocative factors like inflammation, glycation, and oxidative stress. The hypothesis stating the role of RAGE stimulating CSC as a fundamental factor in the initiation and propagation of malignancies concomitant with diabetes has been proposed by Hu and colleagues in diabetics with colon cancer. Pancreatic cancer risk increases by 50% with diabetes mellitus (type 2), which we think can be attributed to the generation of AGEs or glycation of biomolecules in a hyperglycemic niche. Takata and colleagues have reported the induction of pancreatic cancer cell proliferation by AGEs (glyceraldehyde AGEs) [166]. AGEs have displayed pro-tumorigenic effects in breast cancers triggering phosphorylation of ERK 1/2, p38 MAPK, CREB1, and STAT3, augmenting RAGE, and ER expression [4, 18]. All these studies establish the interconnection between diabetes and cancers, mainly of breast (both hormone-dependent and -independent), colon, and pancreas via generation of AGEs and interlinked RAGE signalling axis. Even under non-diabetic conditions, RAGE expression is upregulated in obesity, facilitating adipocyte hypertrophy and affecting insulin sensitivity; these RAGE mediated effects have been attenuated by RAGE siRNA or double knockdown of RAGE ligands—HMGB1 and S100b [167]. As in diabetes, augmentation of RAGE and its ligands predisposes to cancers in obesity also, independent of hyperglycemic status but dependent on dysregulated metabolic niche. This is indicative of the dynamic interplay between RAGE-ligand duo in development of cancers in diabetes and obesity. Hence, we propose the potential stimulation of RAGE and CSC via AGEs and other RAGE ligands as a crucial element promoting tumorigenesis in diseases like diabetes and obesity.
Despite RAGE as a common factor in many diseases like diabetes, cancer, obesity, Alzheimer’s, arthritis, PCOS, certain neuronal, and pulmonary disorders, we suggest that incidence of cancers mainly in diabetic and obese population can be specifically attributed to the co-existing pathological factors like hypoxia, hyperglycemia, oxidative/ carbonyl stress, elevated glycolysis, and inflammatory micromilieu. This setting related with the generation of RAGE ligands stimulates RAGE and CSC by a continuous, self-fuelling loop promoting tumour growth. Re-activation of stem cells otherwise dormant in adults is associated with only one element, i.e. cellular re-growth, which is utilised in a controlled manner in wound healing and uncontrollably in the expansion of cancers [168]. This renewal of CSC by RAGE and its ligands might mark the unique transformation point of diabetes and cancer, which could provide a singular target for the prevention of cancer in diabetics. Diabetes is associated with poor survival of cancer patients [169], and even in non-diabetic patients with advanced stage cancers, elevated blood glucose levels have displayed poor outcome and decreased overall survival [170]. Altogether, as we have observed, diabetes and obesity play a significant role in the onset of cancers, whereas high blood glucose in advanced cancers, irrespective of the diabetic positivity of the patient, affects the patient survival and treatment outcome. This indicates the significance of managing blood glucose levels for the prevention of not only diabetes but also cancer and for improving the patient survival in end stage cancers.
We also emphasise the need for crucial care to be taken in stem cell treatment for diabetes and any other RAGE overexpressing diseases, given the interconnection between RAGE, its ligands, and CSC. The presence of augmented expression of RAGE and its ligands in multiple diseases provides better prospects for developing RAGE blockers and its ligand inactivators, especially in cancers, where the RAGE-ligand duo can be implicated in different stages and hallmarks of different neoplasms. This also makes RAGE-mediated disease targeting plausible since RAGE is highly expressed in innumerable pathological states and hardly under physiological conditions.
Hallmarks of Cancer—Initiation to Invasion—RAGE/Ligands
Hanahan and Weinberg framed ten vital features of tumours as “hallmarks,” comprising of a multitude of biological characteristics adapted by cancer cells through the course of their genesis, progression, and metastasis [171]. It can be noted that RAGE, with its various ligands, is not just a mediator of inflammation or immune reaction. Given the right ligand touch, the receptor has an implication in each of the proposed hallmark of cancer, underlining its emergent role not only in different diseases but also in the different stages and features of cancer. Several hallmarks of cancers have been described and innumerable genes studied for deriving the rightful definition of tumours and curbing the underlying putative mechanism [171]. The downstream signalling cascade fuelled by sustained RAGE-ligand interaction in an inflammatory, hyperglycemic niche under constant oxidative stress, favours the derivation of different hallmarks of cancer (detailed in Fig. 3).
Molecular interplay between RAGE and its ligands directing the subsequent cellular events towards the execution of Hallmarks of Cancer. Each inner circle represents specific hallmark of cancer and the corresponding outer rectangular box depicts the genes or proteins involved in facilitating the particular hallmark upon RAGE stimulus
Altogether, various signalling molecules concerned with RAGE-ligand interaction converge towards the promotion of an array of transcription factors NF-ķB, CREB, HIF-1α, STAT-3, and AP-1, instigating cancer survival, spread, stem cell renewal, and relapse. All the variant factors come together to uphold the metabolic and genomic dysregulation of tumours, favoured by unhindered rise in proliferative indices, molecular insults, and mutational load. A deeper understanding of the underlying molecular pathways points towards the inter-connection between RAGE-ligand signal network and neoplasms and targeting the same for successful onco-therapeutics.
Therapeutic Targeting of RAGE/Ligand Axis—the Epicentre of Genesis and Recurrence of Tumours
Throughout the various scientific approaches and developments made towards a cure of cancer, what makes the disease more challenging to treat and even more difficult to control is its ability to regrow and spread out branches simultaneously while trying to sever its roots with drugs. The process being explained by drug resistance and metastasis, which are the two most demanding aspects of cancer therapeutics, without which eliminating the mortality and morbidity of the disease would have been made possible with just the advent of cytotoxic drugs. The important fact is the aggression and rapidity with which cancers spread and metastasise to distant sites even after stringent chemotherapy and/or radiotherapy regimens. RAGE overexpressing tumours are found to be biologically aggressive with high malignant grade, poor prognosis, and higher inclination to metastasize and recur.
RAGE—Ligand—Cancer Cycle
Metabolic dependency of cancer cells is a major factor determining the aggressive nature of tumours. Tumour cells and/or niche exhibiting Warburg’s type of glycolytic dependency display elevated mitotic and metastatic indices [44]. High glycolytic rate of tumours favour invasion and metastasis through a vicious circle of cellular and molecular events (described in Fig. 4) characterised by hypoxia, hyperglycemia, glycation, and inflammation, involving RAGE directed signalling cascade. The RAGE signal cues lead to self-sustained cyclic events of oncogenic processes, responsible for the onset and propagation of cancers (detailed in “Cancer Metabolism and Glycation—AGEs” and “Glycolytic Dependency and Cancer Malignancy—AGEs and RAGE”). HIF-1α stimulated by intratumoral hypoxia leads to elevated glycolysis and lactate accumulation, by upregulation of proteins linked with glycolysis, cell growth, and glucose transport (“Metabolic Reprogramming and Sustained Growth Signals—AGEs” and “Invasion and Tumour-Associated Inflammation”). Lactate in turn activates Hyaluronan synthesis (dysregulated in cancers), instigates up regulation of HIF-1α, VEGF and enhances motility, creating the acidic micro milieu favouring angiogenesis and metastasis. Besides lactate upsurge, glycolysis and its glycating by-products perpetuate AGE generation, promoting HMGB1 and S100 proteins, evoking RAGE-NF-ķB and sustaining inflammation (explained in section “RAGE/Ligands—Glycation, Inflammation, and Hallmarks of Oncogenesis”). Downstream MAPK and PI3K/AKT pathways are activated mainly, further mounting up HIF-1α expression, thus fuelling the loop leading to tumorigenesis and promotion of cancers (section “Cancer Stem Cells and EMT”).
Cancer can be initiated at any point in the cycle (Fig. 4), leading to an automatic ignition of non-stop tumorigenic events, advancing the tumour grade and aggressive nature, and evading further interference with oncotherapy, unless targeted with combinatorial therapeutics involving synergistic agents. Targeting RAGE and its ligands or MEK or mTOR inhibitors or any other individual targeted therapy alone as such can only serve to arrest the cycle temporarily. But again, it will trigger the vicious circle out of dormancy under favourable conditions, the point of origin being glycation or inflammation, which fuel the tumour in its nascent and resurrected states. Since all RAGE ligands also have RAGE-independent mechanisms of inducing cancers like Toll-like receptors for DAMPs and receptor independent tumorigenic effects of AGEs, this can finally lead to therapy resistance and tumour relapse. Hence, RAGE and its ligands have to be targeted simultaneously for better therapeutic inhibition, alongside conventional chemotherapy to ensure complete impediment of RAGE-ligand signalling mediated induction and propagation of cancers.
RAGE—Ligand-Targeted Therapeutics
We suggest simultaneous targeting of RAGE and its ligands in par with conventional chemotherapy regimen, which could aid in effective control and treatment of neoplasms. This requires the assimilation and assessment of inhibitors of RAGE and its ligands (small molecules, interfering RNAs, monoclonal antibodies, aptamers) studied so far in cancer and other RAGE related diseases and detailed notion of their possible implications in oncotherapeutics (Table 2) along with conventional measures. Utilisation of RAGE antagonists resulted in major blockade of tumour development and metastasis, as seen from various studies [73, 102, 202, 203]. Besides RAGE knockdown, abrogation of HMGB1 and S100 group of proteins has also shown inhibition of tumour growth and spread [89, 90, 110, 112, 204]. Direct RAGE antagonists like azeliragon and telmisartan have been assessed for their efficacy in Alzheimer’s (in phase III clinical trials) [205] and hypertension [206], respectively. Indirect RAGE antagonist, metformin blocks RAGE through its inhibitory activity on AGE-mediated disturbances in cell signalling [207]. These small molecule RAGE inhibitors mainly azeliragon and metformin can be exploited to understand their efficacy in cancer and advanced clinically to facilitate effective abrogation of RAGE and dependent oncogenic processes.
Anti-cancer drugs with RAGE binding affinity can have potential efficacy against cancers and one such drug has been proved to be cytotoxic against Endometrial and ovarian cancer cells (patent) [250]. Moreover, tamoxifen, a breast cancer drug has been found to enhance the endometrial expression of RAGE in breast cancer patients with or without endometrial cancer. This could possibly imply tamoxifen as an inducing factor of endometrial cancer in breast cancer patients and promotion of metastasis in patients already with endometrial cancer. As reported, instigation of RAGE expression by tamoxifen can be mitigated by combining it with an anti-cancer drug, capable of binding and inhibiting RAGE. Methotrexate has been reported to hinder HMGB1 and RAGE interaction by binding HMGB1 in RAGE binding site and down regulate the interaction dependent mitogenic and inflammatory activities [181]. Cytotoxic drugs with similar antagonism against RAGE and/or its ligands can be analysed for plausible implications in effective oncotherapy. Plant derived drugs like curcumin [208], quercetin [194], epigallocatechin gallate [189, 190], and glycyrrhizin [192, 193]; besides, nifedipine [112] and ethyl pyruvate [186] have displayed both HMGB1 inhibiting and anti-cancer effects. Quercetin exerted blockade of RAGE and its both ligands—AGE and HMGB1 expression in breast cancer cells.
AGE-Targeted—Anti-Glycation Agents or AGE Inhibitors
AGEs exhibit a pleiotropic nature in cancer by inducing the expression of RAGE and its other ligands—HMGB1/S100 (detailed in section “Sustained Inflammation and Oncogenic Transformation—AGEs”) and multitude of receptor independent effects (section “RAGE-Independent Effects of AGEs”). Although the importance of AGE inhibitors or anti-glycation agents in treating cancer has not been considered so far, AGE-RAGE interaction plays significant role in proliferation and propagation of different cancers [5, 16, 209]. Hence, we insist the potential advantage of including AGE inhibitors with traditional chemotherapy to enhance the efficacy of the same, taking into consideration the potential participation of AGEs in oncogenesis and metastasis. Different plant-based lead molecules like curcumin [210], quercetin [195], resveratrol [211,212,213], genistein [214, 215], and garcinol [216, 217] have been found to possess both anti-cancer and anti-glycation activities (AGE inhibitors), which could be utilised in the context of AGE targeting in cancer treatment. Of these, resveratrol has been shown to inhibit both AGEs and RAGE in diabetes and effective in cancer management. Several small molecules, aptamers, antibodies, peptibodies, interfering RNAs, capable of inhibiting RAGE and/or its ligands—AGEs, HMGB1, and S100 that have been proved to be effective in cancer through several in vitro and in vivo studies are detailed (Table 2) for plausible translation of their application in clinical oncotherapeutics. In an ongoing clinical trial conducted in Medical University of South Carolina (2017–19), Carolyn Britten and colleagues have attempted to evaluate the effect of metformin and a derivative of grape seed extract on the level of AGEs in metastatic breast cancer and prostate cancer patients to establish the possible relationship between AGEs and cancer and assess the AGEs inhibiting potential of the drugs for implications in cancer treatment (clinical trials) [251, 252]. We studied a novel pharmaceutical formulation of phytopolyphenols—a flavonoid glycoside, diosmin, and a naphthoquinone, plumbagin in MDA-MB-231 cells (TNBC). We obtained promising cytotoxic effects revealing potent synergistic anti-proliferative efficacy of the polyphenols with greater than 80% inhibition, combination index as low as 0.3, even at minimal doses. The combination involved the use of Diosmin displaying anti-glycation activity, for the potentiation of anti-cancer effects of the formulation. We observed enhanced cytotoxic efficacy of the formulation rather than individual drugs, with efficient dose reduction index (DRI > 1) upon inclusion of an anti-glycation agent or AGE inhibitor (diosmin) for the first time (patent) [248]. This formulation unravelled the potent anti-neoplastic efficacy of the drugs targeting glycation/glycoxidation in cancer. The chemo-preventive and ROS scavenging effects of the specified anti-glycation agent might serve to prevent and delay cancer incidence, considering the age, AGEs, and ROS-related degenerative changes and genomic insults to the body, contributing to cancers in the aged and risk population.
Certain antibodies, peptibodies, anti-inflammatory, anti-histamine, anti-protozoal, and quinolone derivatives have been found to be antagonists of different S100 group of proteins (Table 2). S100 group of proteins and AGEs exhibit different types of proteins (S100 A4, S100B, S100P, etc.) and structural diversity (different AGEs depending on glycating agent and glycated molecule), using antibodies against single protein may not target all the isoforms, unlike RAGE and HMGB1. Hence, small molecule inhibitors of AGE formation and general S100 inhibitors could provide a wider coverage for the designated drugs. Although S100 inhibitors have been identified, the long half-life of S100 proteins interferes with attaining the constant and sufficient low levels of protein required to produce the drug effects [87].
Inhibitors of protein translocation across biological membranes have also been suggested as possible novel anti-cancer drugs [218]. AGEs, HMGB1, and S100 proteins formed intracellularly can be secreted into the extracellular space upon stimulation [83, 166, 219], and in turn trigger RAGE, inducing strong upregulation of RAGE downstream pathways. Hence, we state that protein translocation blockers can be utilised for preventing the extracellular release of internal RAGE ligands and further RAGE instigation, in addition to the blockade of extracellular RAGE and its ligands by targeted therapeutics.
Potential Advantages of RAGE—Ligand-Targeted Therapeutics
Adding together all the factors, we have listed the possible advantages of involving RAGE-ligand targeted therapeutics in cancer treatment for achieving better prognosis, effective cancer control, and curbing tumour recurrence.
-
Taking into account the pleiotropic behaviour of AGEs in cancer from initiation to invasion, AGE inhibitors, or anti-glycation agents (via control of glycoxidative damage and genomic insults) can have potential applications in cancer from prevention of its occurrence to the prevention of metastasis and cancer recurrence. RAGE-ligand targeted therapy can be coupled with conventional chemotherapy and/or anti-glycation and anti-inflammatory agents, mainly anti-glycation agents like diosmin, quercetin, resveratrol, genistein, and garcinol meant to control overall cellular events besides the molecular components assisting oncogenic processes.
-
Molecular chaperones can be used along with targeted or conventional chemotherapy, since glycation and mutations result in improperly folded proteins forming aggregates, which encourage strong binding of RAGE by its oligomerization and hence RAGE stimulation; several dysregulated proteins in cancer are targets of HSP-90, a molecular chaperone, known to be mutated and downregulated in many cancers [220].
-
Cytotoxic agents with anti-oxidant activity or administering anti-oxidants following conventional chemotherapy could serve to impede the development of resistance to chemotherapy, by mitigating oxidative stress, which otherwise could by themselves evoke AGE generation, angiogenesis (VEGFR activation by ROS), additional genomic and proteomic damage, evasion of apoptosis, and therapy resistance. Since individual anti-oxidant agents have the paradoxical action of promoting cancer, cytotoxic drugs with inherent anti-oxidant potential such as certain flavonoids can be combined for prevention of drug resistance.
-
Evaluation and development of Receptor (RAGE)/ligand (S100/HMGB1) targeted new cytotoxic lead molecules for effective control of origin, spread, relapse, and drug resistance of tumours. The inflammatory (HMGB1) and ROS molecules released from dying cancer cells following chemo/radiotherapy trigger cancer relapse by promotion of downstream oncogenic signalling via RAGE, TLR, and TGF-β [86]. Therapy resistance elicited by HMGB1 and ROS can be prevented using HMGB1 inhibitors and/or ROS scavengers (anti-oxidants) together with cytotoxic drugs.
-
Cancer associated fibroblasts also express AGEs, HMGB1, and S100 group of proteins, activating RAGE in cancer cells. Combination therapies involving antagonists to RAGE, AGEs, HMGB1, and S100 proteins, along with conventional treatment measures for cancer can provide multi-targeting of cancers with varied genomic signature, minimising drug resistance, tumour recurrence, and improving patient survival. Small molecule inhibitors like curcumin and quercetin, acting against AGEs, RAGE, and HMGB1 can be exploited for their pleiotropic therapeutic effects in synergism with cancer chemotherapy.
-
Targeting RAGE and its ligands offers selective toxicity to the developed anti-neoplastic drugs, thus preventing undesirable side effects to normal cells, as they are expressed in high amounts only under pathological conditions, whereas non-cancerous, homeostatic cellular environment express RAGE and its ligands negligibly; additionally, RAGE over expressing precancerous lesions, tumour stroma, and tumour adjacent normal tissues which originate, fuel, and regenerate cancer, respectively, also can be eradicated.
-
Since RAGE is highly expressed in almost all cancer types and there is a proportionate increase in malignant nature of tumours with its elevated levels, developing an anti-cancer drug target against RAGE can aid in treating different types of cancers, especially those with high metastatic grade like TNBCs, which lack recognised target receptors; thus serve to control aggressive cancers with poor prognosis.
-
Ageing leads to degenerative cellular changes by glycoxidative stress (as AGEs also increase with age) and accumulating mutations by sustained genomic damage, accompanied by weakened immune system, which are the major factors cumulative towards the predisposition of aged/older population to cancer incidence. Highlighting the application of anti-glycation (AGE inhibitors) and anti-oxidant agents as chemo-preventive therapeutics could serve towards healthy ageing, free from fatal malignancies, and increased overall and disease-free survival even after incidence, by curbing the cellular and molecular damages caused by pathological factors as diabetes, obesity, genetic predisposition (BRCA1 etc.,), and physiological factors as ageing. Chemo-preventive approach to cancer susceptibles like diabetics and BRCA1 mutation carriers, as in the case of familial breast or ovarian cancers, could be made possible by administering (preferably plant based) anti-glycation agents even before the onset of cancer (Since AGEs have been reported to up regulate estrogen receptor, besides RAGE, making a diabetic more prone to breast cancer).
-
Immunotherapy is gaining momentum in recent cancer research and targeting RAGE-ligand duo can provide effective immunotherapeutics (since antagonists of downstream targets of RAGE such as CTLA-4 and MDSC are assessed for evoking immune activation), working to unhinge the tumour immune evasion mechanics.
Thus, RAGE and its ligands, AGEs, HMGB1, and S100 group of proteins (S100A4, S100P, S100B, S100A8, and S100A9) serve as potential therapeutic targets for cancer.
RAGE-Ligands and Cancer Diagnostics
Recent discoveries of certain onco-suppressive agents have been found to limit progression of the disease, but are found efficient mainly if diagnosed at the early stages of cancer. Many cancer screening techniques have been adopted for prevention, early detection and effective treatment of the disease, while the tumour is still in budding stage. But the silent, asymptomatic nature of most cancers in their initial and even late stages makes it difficult to diagnose or they remain undetectable until too late to treat. This necessitates the advent of newer biochemical and molecular diagnostics, capable of detecting even small masses of malignant tumours at an early stage with precision and accuracy, specific enough not to pick non-malignant tumours as cancers and sensitive enough not to mistake malignant growths as non-cancerous ones. Hence, we have analysed RAGE and certain RAGE associated components for their efficacy as diagnostic and prognostic biomarkers in different cancers.
RAGE Gene Polymorphism—Cancer Biomarker
Certain polymorphisms of RAGE are associated with cancer risk and disease prognosis, mainly, oral, breast, lung, gastric, hepatocellular, colorectal, and pancreatic cancers (Table 3). Of the 30 polymorphisms reported in RAGE gene, Gly82Ser polymorphism is studied more owing to its location in ligand binding V domain of RAGE. This glycine to serine change at position 82 promotes glycosylation, enhancing the RAGE ligand binding, its activation, thus altering its functions and making the carriers prone to certain cancers, diabetes [234] and inflammation [235].
This G82S polymorphism is related with sensitivity to cancer treatment for thermotherapy in non-small cell lung cancer (NSCLC) [223]. G82S and -374T/SA are most commonly seen RAGE variants with significant impact in cancer risk and invasion. Interplay between specific RAGE gene polymorphisms and environmental mutagens is observed to be a predisposing factor for oral cancer [227]. Besides their role in cancer risk and affecting treatment response, a few genotypic variants of RAGE are also associated with eliciting metastasis [221, 225, 227].Thereby further screening for RAGE gene polymorphisms in cancer patients could serve to predict the cancer risk, early onset, prognosis, tendency to metastasise and response of tumours to different therapies.
While certain gene polymorphisms as rs2070600 (G82S) and rs1800624 (-374T/A) are suggested as potential screening/diagnostic markers and futuristic therapeutic targets [229], the same polymorphism (-374T/A) which decreases the cancer risk in Caucasians [228], is observed to increase the cancer risk in Asians [230]. And the same polymorphism (-374T/A) which confers reduced risk against one cancer (breast cancer), increase the risk for another cancer (lung cancer) [229]. All these observations imply that RAGE gene polymorphisms could be used as genetic markers to diagnose the risk subjects at an early stage. Major challenges of cancer treatment being metastasis and recurrence, screening for most common genotypic variants associated with cancer invasion like G82S, could help assess the metastatic inclination of cancers to enable early drug targeting.
Plasma Soluble RAGE—Cancer Biomarker
sRAGE (soluble RAGE) said to be one of the splice variants of RAGE, lacking trans membrane and cytoplasmic domain; nullifies the actions of RAGE by competitive ligand binding [236]. sRAGE exist in circulation in normally higher levels and supposedly neutralises the RAGE functions. Hence, the increased or decreased levels of sRAGE protect from or make a person prone to cancers and certain age related disorders [237]. Studies report the decreased sRAGE levels in cancer patients compared to the healthy controls and lower sRAGE levels are found to be associated with increased cancer risk [238, 239]. sRAGE levels are inversely correlated with tumour stage and positively correlated with patient prognosis [236, 240,241,242]. Reduced sRAGE levels are related to progression of breast, lung, liver and colorectal cancers. Hence circulating sRAGE could be used as diagnostic and prognostic biomarker for cancer, besides serving as a decoy molecule or defence mechanism in the prevention of RAGE related pathologies like Alzheimer’s, diabetes and cancer.
As seen from several studies, carriers of certain RAGE polymorphism (Table 3) are prone to have lesser plasma soluble RAGE forms, hence making them susceptible to certain cancers. Different RAGE genotypic variants of G82S have been associated with either reduced or elevated plasma sRAGE levels [232, 233, 243]. Further, correlating the plasma levels of sRAGE with existing RAGE genotypic variants of the subject could reveal the cancer risk in unaffected groups and serve to predict therapy response as well as monitor disease prognosis in cancer individuals. Furthermore detailed analysis for different polymorphisms and plasma soluble RAGE forms in various populations and several cancers would warrant in-depth outlook into the application of these polymorphisms in cancer diagnostics and targeted therapeutics.
Tissue RAGE—Cancer Biomarker
RAGE is a significant diagnostic biomarker, considerably elevated in advanced-stage and highly invasive cancers; an important predictor of tumour stage, progression and recurrence in several cancers including oral and breast carcinoma [45, 244]. We have tried to evaluate the significance of RAGE as a biomarker both in non-cancer and cancer subjects.
-
Metastasis and recurrence tend to be the point of failure of most anti-cancer drugs, decreasing the survival rate of cancer patients. Through evaluation of RAGE and its ligands expression in tumour tissues, we can predict the propensity towards metastasis and recurrence in proportionate with its expression, assess the severity of the disease and treat patients with RAGE-ligand specific antagonists. For cancer survivors or those in remission, constant monitoring for RAGE expression, sRAGE levels and AGEs could help monitor the onset of secondary cancers or cancer relapse.
-
Significant RAGE overexpression in cancer tissues compared to normal adjacent tissues, indicates the diagnostic role of RAGE in cancers. RAGE expression has been reported to increase parallel with the histological grade, tumour size, stage (nodal and metastatic status, AJCC stage), invasive depth and local recurrence [46]. Substantial augmentation of RAGE expression has been observed in aggressive, advanced-stage, metastatic tumours and node-positive tissues compared with other tissues [45]. Hence RAGE-ligand antagonists could serve to treat not only primary but also the undiagnosed metastatic cancers, preventing recurrence [29, 47].
-
The multi-characteristic features of RAGE incorporates its role in different stages and hallmarks of cancer; entails it as a significant biomarker for diagnosis and prognosis of cancers. Highly elevated RAGE expression is a diagnostic as well as a prognostic biomarker to monitor the efficacy of chemotherapy in controlling tumour growth and spread. Upregulated RAGE expression especially in the invasive cancer front implies its prominent role in metastasis and blockade of the same for prevention of metastasis and further disease progression [244].
-
Subdivision within tumour stages can be made based on the expression levels of RAGE in tumour tissues. Late stage cancers without RAGE over expression is possibly indicative of less propensity towards metastasis, local recurrence and better patient survival. Whereas with high RAGE expression in late stages, possibly imply the requirement of (RAGE-ligand) targeted agents and intensive chemotherapy for curbing metastasis, relapse and improving patient survival. High RAGE expression in stage 3 and 4 cancers have revealed overall poor patient survival [245]. When using RAGE blockers, the effectiveness of the treatment and prognosis of the disease can be scrutinised by screening for RAGE expression levels before and after the treatment.
Hence, we propose RAGE could be an efficient diagnostic and independent prognostic biomarker for cancers.
Serum and Tissue AGEs—Cancer Biomarker
-
Upregulation of AGEs in normal tissues surrounding tumours (mentioned in “RAGE-Dependent Effects of AGEs”) suggests the involvement of AGEs in cancer genesis via the stimulation of RAGE expression later leading to tumour progression.
-
Accumulation of AGEs and subsequent RAGE signalling increase cancer risk [9]. Circulating AGE modified protein (argpyrimidine and CML) and DNA (CEdG) can be elucidated as biomarkers for early screening and detection of cancers.
-
Glycated adducts of bio macromolecules in circulation can also be estimated for potential carcinogenic injury to the body system in susceptible individuals, such as those exposed to harmful radiation and chemical carcinogens.
-
Autoantibodies to glycated conjugates of biomolecules can act as valid estimates of glycoxidative damage and possible indicators of abnormal cell metabolism during silent oncogenesis.
-
In people susceptible to cancer such as BRCA1 mutation carriers and people with diabetes, constant screening for greater than normal elevated levels of CEdG, argpyrimidine, CML, and S100 proteins in circulation may reveal the cancer in the early stages.
-
Autofluorescent property of AGEs (Argpyrimidine) can be made use of by checking for their accumulation in mammary glands as a routine health check-up, e.g. mammogram, every year to rule out breast cancer; similarly auto fluorescence technique can be used for assessing skin cancer (melanoma), which can be a non-invasive method compared to screening blood for biomarkers.
-
Other non-invasive methods of measuring AGEs include screening tears, saliva, and urine of patients for prognostic significance, by spectrofluorimetry and ELISA techniques.
-
S100 proteins, mainly S100P, S100A8, and S100A9 are significant diagnostic and prognostic biomarkers of breast cancer, urothelial, hepatocellular, renal, colon, and other cancers [177, 178, 246, 247]. Hence, screening for S100 proteins could help in prompt diagnosis of cancers and prognosis of cancers and to evaluate the tumour stage and drug responsiveness.
RAGE Polymorphism, sRAGE, RAGE, and AGEs—the (S)quad of Cancer Biomarkers
The expression of RAGE and AGEs is proportionate with the malignant and metastatic grade of cancers. Additionally, sRAGE levels and RAGE polymorphisms indicate the susceptibility of a person to specific cancers. The increasing sRAGE levels with RAGE targeted/conventional chemotherapy indicate better prognosis.
Hence in progressed cancers and at any cancer stage, screening for the levels of serum AGEs, plasma soluble RAGE, RAGE in tumour tissues, and existing RAGE gene polymorphisms (82G/S, -374T/A) can help to assess the degree of severity of the cancer, its propensity towards metastasis and the patient’s responsiveness to treatment.
Altogether, the four key components associated with RAGE namely, RAGE polymorphisms, sRAGE, AGEs, and tissue RAGE, can be effectively exploited as novel diagnostic and prognostic biomarkers in cancer. Investigating and correlating the factors of “four” in both cancer and non-cancer subjects can have prospective implications, explained as follows:
-
To predict the predisposition to cancer incidence
-
To diagnose cancers at an early stage
-
To monitor the prognosis of primary cancers
-
To evaluate the efficacy of different oncotherapeutic agents
-
To predict the propensity towards metastasis and recurrence
-
To prevent the secondary or metastatic cancers
-
To treat the undiagnosed metastatic cancers alongside primary cancers
-
To assess the overall and disease-free patient survival
-
To evaluate the need for use of RAGE-ligand targeted therapy, alongside conventional therapies for cancer.
It is notable that except RAGE gene polymorphism, all the other three can be altered with specific drug treatments or measures; such as AGEs with anti-glycation agents or AGE-inhibitors, plasma sRAGE, and tumour RAGE with RAGE-targeted therapeutic agents. Hence, screening for this RAGE (s)quad of biomarkers could enable preventing the cancer incidence and progression to metastasis and relapse, at early stages with targeted treatment. We have added together the existing and novel upsides of exploiting RAGE-ligand antagonists and RAGE (s)quad of therapeutic targets for controlling the onset and expansion of cancers.
Conclusion
Developing RAGE-ligand targeted therapeutics and diagnostics can serve to treat and diagnose cancers in a better way. Combining cancer chemotherapy with inhibitors of RAGE, AGEs, and other RAGE ligands could provide multi-dimensional approach for curbing tumours with varied genomic architecture, since abnormal growths do not always have a uniform genomic profile. RAGE-ligand signalling network plays significant role in each of the hallmark of cancer, commencing from origin to spread (Figs. 1–3). RAGE-ligand antagonists could significantly target the Achilles heel of cancers mainly, poor prognosis, silent metastasis, drug resistance, and cancer recurrence, since blockade of RAGE or HMGB1 or S100 proteins alone have exhibited considerable reduction in tumour size, invasion and angiogenesis of several cancers [73, 89] (Table 2). Of all, overall and disease-free survival of cancer patients can be extended to confer them with better quality of life, while simultaneously trying to mitigate the morbidity and mortality. Although various mechanisms have been coined, timely detection and treatment of cancers still remain elusive and the field remains still as ongoing research. Proper utilisation of the (s)quad of RAGE-ligands and RAGE-biomarkers for appropriate sorting of neoplasms from normal cells at the budding stage could make cancers more curable and controllable. Deeper knowledge of the neoplastic molecular mechanisms so derived needs to go hand in hand with translational implications for patient benefits. Transforming book information to a laboratory test tube and deciphering the same for a live tumour milieu requires the convergence of different expertise towards the expansion of targeted and combinatorial therapies and liable screening techniques for improved detection and management of cancers in future. In this review, we have attempted to give a prelude to the same.
Abbreviations
- AGEs:
-
advanced glycation end products
- AP-1:
-
activator protein-1
- AKT:
-
protein kinase B
- COX-2:
-
cyclo-oxygenase-2
- CREB:
-
cAMP response element-binding protein
- CTLA4:
-
cytotoxic T-lymphocyte-associated protein 4
- CXCR4:
-
chemokine receptor type 4
- Dia-1:
-
diaphanous-1
- EGFR:
-
epidermal growth factor receptor
- EMT:
-
epithelial mesenchymal transition
- ER:
-
estrogen receptor
- ERK:
-
extracellular signal-regulated kinase
- GLUT-1:
-
glucose transporter-1
- GSK-3β:
-
glycogen synthase kinase- 3 β
- HIF-1α:
-
hypoxia inducible factor-1
- hTERT:
-
telomerase reverse transcriptase
- ICAM:
-
intercellular adhesion molecule
- JAK:
-
janus kinase
- JNK:
-
c-Jun NH2-terminal kinase
- HMGB1:
-
highmobility group box 1 protein
- MAPK:
-
mitogen-activated protein kinase
- MDSCs:
-
myeloid-derived suppressor cells
- MEK:
-
MAPK/ERK kinase
- MMP:
-
matrix metallo proteinases.
- mTOR:
-
mammalian target of rapamycin
- NADPH oxidase:
-
nicotinamide adenine dinucleotide phosphate oxidase
- NF-ķB:
-
nuclear factor-ķB
- NOX:
-
NADPH oxidase
- OPN:
-
osteopontin
- PARP:
-
poly-ADP ribose polymerase
- PGE2:
-
prostaglandin E2
- PI3K:
-
phosphatidyl inositol-3-kinase
- PRR:
-
pattern recognition receptor
- PTEN:
-
phosphatase and tensin homolog protein
- P70S6K:
-
70-kDa ribosomal protein S6 kinase
- RAGE:
-
receptor for advanced glycation end products
- RAS:
-
small GTPase binding protein
- Rb:
-
retinoblastoma protein
- ROCK:
-
rho-associated, coiled-coil-containing protein kinase 1
- SP-1:
-
stimulator protein-1
- STAT:
-
signal transducers and activators of transcription
- TGF-β:
-
transforming growth factor-β
- TNF-α:
-
tumour necrosis factor- α
- VCAM:
-
vascular cell adhesion molecule
- VEGF:
-
vascular endothelial growth factor
- VEGFR2:
-
vascular endothelial growth factor receptor 2
References
World Health Organization. Breast cancer estimated incidence, mortality and prevalence worldwide in 2012. 2012. URL: http://globocan iarc fr/old/FactSheets/cancers/breast-new asp [WebCite Cache ID 6aKrqTle9]
Mathupala SP, Ko YH, Pedersen PL (2010) The pivotal roles of mitochondria in cancer: Warburg and beyond and encouraging prospects for effective therapies. BBA-Bioenergetics 1797(6):1225–1230
Ryu TY, Park J, Scherer PE (2014) Hyperglycemia as a risk factor for cancer progression. Diabetes Metab J 38(5):330–336
Sharaf H, Matou-Nasri S, Wang Q, Rabhan Z, Al-Eidi H, Al Abdulrahman A, Ahmed N (2015) Advanced glycation endproducts increase proliferation, migration and invasion of the breast cancer cell line MDA-MB-231. Biochim Biophys Acta 1852(3):429–441. https://doi.org/10.1016/j.bbadis.2014.12.009
Turner DP (2015) Advanced glycation end-products: a biological consequence of lifestyle contributing to cancer disparity. Cancer Res 75(10):1925–1929
Heijst JWJ, Niessen HWM, Hoekman K, Schalkwijk CG (2005) Advanced glycation end products in human cancer tissues: detection of Nε-(carboxymethyl) lysine and argpyrimidine. Ann N Y Acad Sci 1043(1):725–733
Ashraf JM, Shahab U, Tabrez S, Lee EJ, Choi I, Yusuf MA, Ahmad S (2016) DNA glycation from 3-deoxyglucosone leads to the formation of AGEs: potential role in cancer auto-antibodies. Cell Biochem Biophys 74(1):67–77
Synold T, Xi B, Wuenschell GE, Tamae D, Figarola JL, Rahbar S, Termini J (2008) Advanced glycation end products of DNA: quantification of N 2-(1-carboxyethyl)-2′-deoxyguanosine in biological samples by liquid chromatography electrospray ionization tandem mass spectrometry. Chem Res Toxicol 21(11):2148–2155
Yamagishi S, Matsui T, Fukami K (2015) Role of receptor for advanced glycation end products (RAGE) and its ligands in cancer risk. Rejuvenation Res 18(1):48–56
Logsdon CD, Fuentes MK, Huang EH, Arumugam T (2007) RAGE and RAGE ligands in cancer. Curr Mol Med 7(8):777–789
Rahimi F, Karimi J, Goodarzi MT, Saidijam M, Khodadadi I, Razavi AN, Nankali M (2017) Overexpression of receptor for advanced glycation end products (RAGE) in ovarian cancer. Cancer Biomark 18(1):61–68. https://doi.org/10.3233/cbm-160674
Tamae D, Lim P, Wuenschell GE, Termini J (2011) Mutagenesis and repair induced by the DNA advanced glycation end product N2-1-(carboxyethyl)-2′-deoxyguanosine in human cells. Biochemistry 50(12):2321–2329. https://doi.org/10.1021/bi101933p
Wei Y, Chen L, Chen J, Ge L, He RQ (2009) Rapid glycation with D-ribose induces globular amyloid-like aggregations of BSA with high cytotoxicity to SH-SY5Y cells. BMC Cell Biol 10(1):10
Baraka-Vidot J, Guerin-Dubourg A, Bourdon E, Rondeau P (2012) Impaired drug-binding capacities of in vitro and in vivo glycated albumin. Biochimie 94(9):1960–1967
Ansari NA, Ali R (2011) Physicochemical analysis of poly-L-lysine: an insight into the changes induced in lysine residues of proteins on modification with glucose. IUBMB Life 63(1):26–29
Bao J-M, He M-Y, Liu Y-W, Lu Y-J, Hong Y-Q, Luo H-H, Ren Z-L, Zhao S-C, Jiang Y (2015) AGE/RAGE/Akt pathway contributes to prostate cancer cell proliferation by promoting Rb phosphorylation and degradation. Am J Cancer Res 5(5):1741
Yaser AM, Huang Y, Zhou RR, Hu GS, Xiao MF, Huang ZB, Duan CJ, Tian W, Tang DL, Fan XG (2012) The role of receptor for advanced glycation end products (RAGE) in the proliferation of hepatocellular carcinoma. Int J Mol Sci 13(5):5982–5997
Matou-Nasri S, Sharaf H, Wang Q, Almobadel N, Rabhan Z, Al-Eidi H, Yahya WB, Trivilegio T, Ali R, Al-Shanti N, Ahmed N (2017) Biological impact of advanced glycation endproducts on estrogen receptor-positive MCF-7 breast cancer cells. Biochim Biophys Acta (BBA) Mol Basis Dis 1863(11):2808–2820
Fritz G (2011) RAGE: a single receptor fits multiple ligands. Trends Biochem Sci 36(12):625–632
Xue J, Rai V, Singer D, Chabierski S, Xie J, Reverdatto S, Burz David S, Schmidt AM, Hoffmann R, Shekhtman A (2011) Advanced glycation end product recognition by the receptor for AGEs. Structure 19(5):722–732
Cai W, Ramdas M, Zhu L, Chen X, Striker GE, Vlassara H (2012) Oral advanced glycation endproducts (AGEs) promote insulin resistance and diabetes by depleting the antioxidant defenses AGE receptor-1 and sirtuin 1. Proc Natl Acad Sci 109(39):15888–15893
Nedić O, Rattan SIS, Grune T, Trougakos IP (2013) Molecular effects of advanced glycation end products on cell signalling pathways, ageing and pathophysiology. Free Radic Res 47(sup1):28–38
Ramasamy R, Vannucci SJ, Yan SS, Herold K, Yan SF, Schmidt AM (2005) Advanced glycation end products and RAGE: a common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology 15(7):16R–28R
Srikanth V, Maczurek A, Phan T, Steele M, Westcott B, Juskiw D, Münch G (2011) Advanced glycation endproducts and their receptor RAGE in Alzheimer’s disease. Neurobiol Aging 32(5):763–777
Diamanti-Kandarakis E, Piperi C, Patsouris E, Korkolopoulou P, Panidis D, Pawelczyk L, Papavassiliou AG, Duleba AJ (2007) Immunohistochemical localization of advanced glycation end-products (AGEs) and their receptor (RAGE) in polycystic and normal ovaries. Histochem Cell Biol 127(6):581–589
Gomes R, Silva MS, Quintas A, Cordeiro C, Freire A, Pereira P, Martins A, Monteiro E, Barroso E, Freire AP (2005) Argpyrimidine, a methylglyoxal-derived advanced glycation end-product in familial amyloidotic polyneuropathy. Biochem J 385(2):339–345
Xie J, Mendez JD, Mendez-Valenzuela V, Aguilar-Hernandez MM (2013) Cellular signalling of the receptor for advanced glycation end products (RAGE). Cell Signal 25(11):2185–2197. https://doi.org/10.1016/j.cellsig.2013.06.013
Ko S-Y, Ko H-A, Shieh T-M, Chang W-C, Chen H-I, Chang S-S, Lin I-H (2014) Cell migration is regulated by AGE-RAGE interaction in human oral cancer cells in vitro. PLoS One 9(10):e110542
Nasser MW, Wani NA, Ahirwar DK, Powell CA, Ravi J, Elbaz M, Zhao H, Padilla L, Zhang X, Shilo K (2015) RAGE mediates S100A7-induced breast cancer growth and metastasis by modulating the tumor microenvironment. Cancer Res 75(6):974–985
Kang R, Hou W, Zhang Q, Chen R, Lee YJ, Bartlett DL, Lotze MT, Tang D, Zeh HJ (2014) RAGE is essential for oncogenic KRAS-mediated hypoxic signaling in pancreatic cancer. Cell Death Dis 5(10):e1480
Hiba Z, Borden Katherine LB (2013) Mechanisms and insights into drug resistance in cancer. Front Pharmacol 4
Housman G, Byler S, Heerboth S, Lapinska K, Longacre M, Snyder N, Sarkar S (2014) Drug resistance in cancer: an overview. Cancers 6(3):1769–1792
Shi W, Jiang T, Nuciforo P, Hatzis C, Holmes E, Harbeck N, Sotiriou C, Peña L, Loi S, Rosa DD (2017) Pathway level alterations rather than mutations in single genes predict response to HER2-targeted therapies in the neo-ALTTO trial. Ann Oncol 28(1):128–135
Sullivan Lucas B, Gui Dan Y, Vander Heiden Matthew G (2016) Altered metabolite levels in cancer: implications for tumour biology and cancer therapy. Nat Rev Cancer 16(11):680–693
Pelicano H, Dai J, Liu J, Pusztai L, Hammoudi N, Huang P, Xu R-H, Zhang W (2014) Mitochondrial dysfunction in some triple-negative breast cancer cell lines: role of mTOR pathway and therapeutic potential. Breast Cancer Res 16(5):434
Fais S, Venturi G, Gatenby B (2014) Microenvironmental acidosis in carcinogenesis and metastases: new strategies in prevention and therapy. Cancer Metastasis Rev 33(4):1095–1108
Kellenberger LD, Bruin JE, Greenaway J, Campbell NE, Moorehead RA, Holloway AC, Petrik J (2010) The role of dysregulated glucose metabolism in epithelial ovarian cancer. J Oncol:2010
Eales KL, Hollinshead KER, Tennant DA (2016) Hypoxia and metabolic adaptation of cancer cells. Oncogenesis 5(1):e190
Gatenby Robert A, Gawlinski Edward T (2003) The glycolytic phenotype in carcinogenesis and tumor invasion. Cancer Res 63(14):3847–3854
Ansari NA, Rasheed Z (2009) Non-enzymatic glycation of proteins: from diabetes to cancer. Biochem (Mosc) Suppl Ser B Biomed Chem 3(4):335–342
Poulsen MW, Hedegaard RV, Andersen JM, de Courten B, Bügel S, Nielsen J, Skibsted LH, Dragsted LO (2013) Advanced glycation endproducts in food and their effects on health. Food Chem Toxicol 60:10–37
Claudia L-C, Karen C-N (2010) Dietary advanced glycation end products and aging. Nutrients 2(12):1247–1265
Dion F, Laura S, Walter Katherine R, Nogueira Lourdes M, Hleb F, Turner Ryan Y, Mahtabuddin A, Salley Judith D, Ford Marvella E, Findlay VJ (2014) AGE metabolites: a biomarker linked to cancer disparity? Cancer Epidemiol Biomarkers Prev 23(10):2186–2191
Junjeong C, Do Hee K, Hee JW, Seung KJ (2013) Metabolic interaction between cancer cells and stromal cells according to breast cancer molecular subtype. Breast Cancer Res 15(5):R78
Nankali M, Karimi J, Goodarzi MT, Saidijam M, Khodadadi I, Razavi AN, Rahimi F (2016) Increased expression of the receptor for advanced glycation end-products (RAGE) is associated with advanced breast cancer stage. Oncol Res Treat 39(10):622–628. https://doi.org/10.1159/000449326
Da W, Tingting L, Gengtai Y, Zhiyong S, Yanfeng H, Tingyu M, Jiang Y, Sihao L, Hao L, Guoxin L (2015) Overexpression of the receptor for advanced glycation endproducts (RAGE) is associated with poor prognosis in gastric cancer. PLoS One 10(4):e0122697
Selvia S, Tarek A, Amr A (2017) Differentiation of RAGE expression in primary tumors and lymph node metastatic deposits of breast carcinoma. Imp J Interdiscip Res 3(6)
Korwar Arvind M, Bhonsle Hemangi S, Chougale Ashok D, Kote Sachin S, Gawai Kachru R, Ghole Vikram S, Koppikar Chaitanyananda B, Kulkarni Mahesh J (2012) Analysis of AGE modified proteins and RAGE expression in HER2/neu negative invasive ductal carcinoma. Biochem Biophys Res Commun 419(3):490–494
Bing P, Hui R, Yijing M, Donghui L, Baoqi Y, Liang J, Ling P, Jing L, Liangui Y, Xiaofeng L (2012) High-density lipoprotein of patients with type 2 diabetes mellitus elevates the capability of promoting migration and invasion of breast cancer cells. Int J Cancer 131(1):70–82
Jin PL, Lin L, Qi Z, Yan ZR, Feng SW, Ying S (2012) Increased blood glycohemoglobin A1c levels lead to overestimation of arterial oxygen saturation by pulse oximetry in patients with type 2 diabetes. Cardiovasc Diabetol 11(1):110
Philippe R, Emmanuel B (2011) The glycation of albumin: structural and functional impacts. Biochimie 93(4):645–658
Rouf MA, Safia H, Farzana K, Khursheed A, Asif A (2015) Structural changes in histone H2A by methylglyoxal generate highly immunogenic amorphous aggregates with implications in auto-immune response in cancer. Glycobiology 26(2):129–141
Saheem A, Kiran D, Uzma S, Khursheed A, Asif A (2011) Genotoxicity and immunogenicity of DNA-advanced glycation end products formed by methylglyoxal and lysine in presence of Cu2+. Biochem Biophys Res Commun 407(3):568–574
Yamagishi S (2011) Role of advanced glycation end products (AGEs) and receptor for AGEs (RAGE) in vascular damage in diabetes. Exp Gerontol 46(4):217–224. https://doi.org/10.1016/j.exger.2010.11.007
Uetz-von AE, Michael K, Günter F, Legler DF (2008) V domain of RAGE interacts with AGEs on prostate carcinoma cells. Prostate 68(7):748–758
Rabbani Naila, Thornalley Paul J (2014). Dicarbonyl proteome and genome damage in metabolic and vascular disease. Biochem Soc Trans.
Deng R, Wu H, Hui R, Kong X, Hu L, Wang X, Qing S (2017) Glucose-derived AGEs promote migration and invasion of colorectal cancer by up-regulating Sp1 expression. BBA-Gen Subjects 1861(5):1065–1074
Shun-Yao K, Hshin-An K, Tzong-Ming S, Tzong-Cherng C, Chen H-I, Yi-Ting C, Yu Y-H, Yang S-H, Shu-Shing C (2017) Advanced glycation end products influence oral cancer cell survival via Bcl-xl and Nrf-2 regulation in vitro. Oncol Lett 13(5):3328–3334
Jun-Ichi T, Sho-Ichi Y, Masayoshi T (2010) Cancer malignancy is enhanced by glyceraldehyde-derived advanced glycation end-products. J Oncol:2010
Riichiro A, Sho-ichi Y (2008) AGE-RAGE system and carcinogenesis. Curr Pharm Des 14(10):940–945
Lin J-A, Wu C-H, Gow-Chin Y (2017) Methylglyoxal displays colorectal cancer-promoting properties in the murine models of azoxymethane and CT26 isografts. Free Radic Biol Med
Dachun Y, Michael B (2010) Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes 59(1):249–255
Kuang CY, Rusliza B, Herni T, Hing TT, Norshariza N (2013) Receptor for advanced glycation end products and its involvement in inflammatory diseases. Int J Inflamm:2013
Christiane O, Kathleen J, Elisa H, Navarrete SA, Tilman G, Andreas S (2014) Role of advanced glycation end products in cellular signaling. Redox Biol 2:411–429
Christoffer G, Astrid R, Moritz D, Julia N, Gerhard F, Karin M-D, Alexander E, Bernd A, Bierhaus Angelika NPP (2008) RAGE signaling sustains inflammation and promotes tumor development. J Exp Med 205(2):275–285
Barbel L-S, Markus S, Peter N, Angelika B (2007) RAGE signaling in cell adhesion and inflammation. Curr Pediatr Rev 3(1):1–9
Sparvero Louis J, Denise A-A, Rui K, Daolin T, Neilay A, Jaehyun I, Ronnye R, Lin Brenda AAA, Zeh Herbert J (2009) RAGE (Receptor for Advanced Glycation endproducts), RAGE ligands, and their role in cancer and inflammation. J Transl Med 7(1):17
Martinez-Outschoorn Ubaldo E, Curry Joseph M, Ying-Hui K, Lin Z, Madalina T, David C, Birbe Ruth C, Edmund P, Alessandro B, Pestell RG (2013) Oncogenes and inflammation rewire host energy metabolism in the tumor microenvironment: RAS and NFκB target stromal MCT4. Cell Cycle 12(16):2580–2597
Masson Norma RPJ (2014) Hypoxia signaling pathways in cancer metabolism: the importance of co-selecting interconnected physiological pathways. Cancer Metab 2(1):3
Sina G, Christian V, Philipp S, Doris M (2012) Hypoxia and HIF-1 increase S100A8 and S100A9 expression in prostate cancer. Int J Cancer 131(12):2785–2794
Masuko U-F, Yoshimasa N (2008) Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy. Cancer Lett 266(1):37–52
Xuebo C, Leying Z, Zhang Ian Y, Junling L, Huaqing W, Mao O, Shihua W, Carvalho d FAC, Lihong W, Yasuhiko Y (2014) RAGE expression in tumor-associated macrophages promotes angiogenesis in glioma. Cancer Res 74(24):7285–7297
Huasheng L, Yuhua Z, Shaobi Z, Liang P (2011) Knockdown of RAGE expression inhibits colorectal cancer cell invasion and suppresses angiogenesis in vitro and in vivo. Cancer Lett 313(1):91–98
Wang W, Luo H-S, Yu B-P (2004) Expression of NF-κB and human telomerase reverse transcriptase in gastric cancer and precancerous lesions. World J Gastroenterol 10(2):177
Masaharu A, Teru H, Toshiaki H, Yu-Tzu T, Mitsiades Constantine S, Nicholas M, Dharminder C, Paul R, Munshi Nikhil C, Anderson Kenneth C (2003) Nuclear factor-κB p65 mediates tumor necrosis factor α-induced nuclear translocation of telomerase reverse transcriptase protein. Cancer Res 63(1):18–21
Chung LK, Vinay T (2013) Telomerase: central regulator of all of the hallmarks of cancer. Trends Biochem Sci 38(9):426–434
Lipinska Natalia, Romaniuk Aleksandra, Paszel-Jaworska Anna, Toton Ewa, Kopczynski Przemyslaw, Rubis Blazej. 2017. Telomerase and drug resistance in cancer. Cell Mol Life Sci:1–12
Wang Julie C, Bennett Martin R. 2010. Nuclear factor-κΒ-mediated regulation of telomerase. Am Heart Assoc
Ghosh A, Saginc G, Leow SC, Khattar E, Shin EM, Yan TD, Wong M, Zhang Z, Li G, Sung WK, Zhou J, Chng WJ, Li S, Liu E, Tergaonkar V (2012) Telomerase directly regulates NF-kappaB-dependent transcription. Nat Cell Biol 14(12):1270–1281. https://doi.org/10.1038/ncb2621
Julio M, Li L, Fattah Farjana J, Ying D, Bey Erik A, Malina P, Jinming G, Boothman David A (2014) Review of poly(ADP-ribose) polymerase (PARP) mechanisms of action and rationale for targeting in cancer and other diseases. Crit Rev Eukaryot Gene Expr 24(1)
Srikrishna Geetha FHH (2009) Endogenous damage-associated molecular pattern molecules at the crossroads of inflammation and cancer. Neoplasia 11(7):615–628
Dara D, Wei-Xing Z, Thompson Craig B (2007) Activation of poly (ADP)-ribose polymerase (PARP-1) induces release of the pro-inflammatory mediator HMGB1 from the nucleus. J Biol Chem 282(24):17845–17854
Kang Rui, Xie Yangchun, Zhang Qiuhong, Hou Wen, Jiang Qingping, Zhu Shan, Liu Jinbao, Zeng Dexing, Wang Haichao, Bartlett David L. 2017. Intracellular HMGB1 as a novel tumor suppressor of pancreatic cancer. Cell Res
Rui K, Qiuhong Z, Zeh Herbert J, Lotze Michael T, Daolin T (2013) HMGB1 in cancer: good, bad, or both? Clin Cancer Res 19(15):4046–4057
Rui K, Daolin T, Schapiro Nicole E, Livesey Kristen M, Adam F, Patricia L, Angelika B, Lotze Michael T, Zeh Herbert J (2010) The receptor for advanced glycation end products (RAGE) sustains autophagy and limits apoptosis, promoting pancreatic tumor cell survival. Cell Death Differ 17(4):666–676
Leclerc Estelle, Vetter Stefan W. 2015. The role of S100 proteins and their receptor RAGE in pancreatic cancer. BBA-Mol Basis Dis 1852 (12):2706–2711
Bresnick Anne R, Weber David J, Zimmer Danna B (2015) S100 proteins in cancer. Nat Rev Cancer 15(2):96–109
Chonggao Y, Hongli L, Baogang Z, Yuqing L, Guohua L, Shijun L, Lei S, Yueliang Q, Xiaolong L, Weiyi C (2013) RAGE-binding S100A8/A9 promotes the migration and invasion of human breast cancer cells through actin polymerization and epithelial–mesenchymal transition. Breast Cancer Res Treat 142(2):297–309
Akihiko T, Blood David C, del Toro G, Anthony C, Lee Daniel C, Qu W, Nozomu T, Yan L, Evanthia L, Caifeng F (2000) Blockade of RAGE–amphoterin signalling suppresses tumour growth and metastases. Nature 405(6784):354–360
Dakhel S, Padilla L, Adan J, Masa M, Martinez JM, Roque L, Coll T, Hervas R, Calvis C, Messeguer R (2014) S100P antibody-mediated therapy as a new promising strategy for the treatment of pancreatic cancer. Oncogenesis 3(3):e92
Hudson BI, Kalea AZ, del Mar AM, Harja E, Boulanger E, Vivette D'A, Schmidt AM (2008) Interaction of the RAGE cytoplasmic domain with diaphanous-1 is required for ligand-stimulated cellular migration through activation of Rac1 and Cdc42. J Biol Chem 283(49):34457–34468
Martini M, De Santis MC, Braccinik L, Gulluni F, Hirsch E (2014) PI3K/AKT signaling pathway and cancer: an updated review. Ann Med 46(6):372–383
AL-Madhagi R, AL-Madhagi Y, Ma X, Juan Z, Cejun Y, Qiong D, Pengfei R, Ye B, Sheng L, Wei W (2013) Specific siRNA targeting receptor for advanced glycation end products (RAGE) decreases proliferation in human breast cancer cell lines. Int J Mol Sci 14(4):7959–7978
Roberts Patrick J, Der Channing J (2007) Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 26(22):3291–3310
Sylvain M, Jacques P (2007) The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1-to S-phase transition. Oncogene 26(22):3227–3239
San-Cher C, Jinn-Yuh G, Chi-Ching H, Shean-Jaw C, Tai-Du L, Yu-Ming K, Jau-Shyang H, Yu-Lin Y, Lea-Yea C (2010) Advanced glycation end-products activate extracellular signal-regulated kinase via the oxidative stress-EGF receptor pathway in renal fibroblasts. Journal of Cellular Biochemistry 109(1):38–48
Qiu-Yu Z, Lin-Qing W, Zhang T, Yan-Fei H, Xu L (2015) Autophagy-mediated HMGB1 release promotes gastric cancer cell survival via RAGE activation of extracellular signal-regulated kinases 1/2. Oncol Rep 33(4):1630–1638
Thiruvengadam A, Logsdon Craig D (2011) S100P: a novel therapeutic target for cancer. Amino Acids 41(4):893–899
Holger Z, Stephan B, Thomas H, Jochen H, Oliver F, Michael L, Bernd W, Erbersdobler Helmut F, Sabine S, Busch Andreas E (2003) RAGE-mediated MAPK activation by food-derived AGE and non-AGE products. Biochem Biophys Res Commun 300(2):311–315
Kwak Taekyoung D-EK, Ergonul A, Miller PC, Braley A, Hwang GH, Zhao D, Besser A, Yamamoto Y, Yamamoto H (2017) Targeting of RAGE-ligand signaling impairs breast cancer cell invasion and metastasis. Oncogene 36(11):1559–1572
Indira E, Sivasakthivel T, Aoshuang C, Guoxing Z, Bosland Maarten C, André K-B, Munirathinam G (2012) Targeting receptor for advanced glycation end products (RAGE) expression induces apoptosis and inhibits prostate tumor growth. Biochem Biophys Res Commun 417(4):1133–1138
Ganju Ramesh K. 2013. Receptor for advanced glycation end products (RAGE) as a novel target for inhibiting breast cancer bone metastasis. Ohio state univ columbus
Fara B-M, Simone F, Steven C, Rebecca M, Jelmar Q, Erika F-D, Plumb Darren A, Leila Z, Patrycja G, Gianmaria L (2016) PIM1 kinase regulates cell death, tumor growth and chemotherapy response in triple-negative breast cancer. Nat Med 22(11):1303–1313
Hardaway Aimalie L, Izabela P (2013) IL-1β, RAGE and FABP4: targeting the dynamic trio in metabolic inflammation and related pathologies. Future Med Chem 5(10):1089–1108
Hai Ping P, Feng Bo T, Li L, Nan Hui Y, Hong Z (2016) IL-1beta/NF-kb signaling promotes colorectal cancer cell growth through miR-181a/PTEN axis. Arch Biochem Biophys 604:20–26. https://doi.org/10.1016/j.abb.2016.06.001
Matsubara D, Niki T, Ishikawa S, Goto A, Ohara E, Yokomizo T, Heizmann CW, Aburatani H, Moriyama S, Moriyama H, Nishimura Y, Funata N, Fukayama M (2005) Differential expression of S100A2 and S100A4 in lung adenocarcinomas: clinicopathological significance, relationship to p53 and identification of their target genes. Cancer Sci 96(12):844–857. https://doi.org/10.1111/j.1349-7006.2005.00121.x
Mueller A, Schafer BW, Ferrari S, Weibel M, Makek M, Hochli M, Heizmann CW (2005) The calcium-binding protein S100A2 interacts with p53 and modulates its transcriptional activity. J Biol Chem 280(32):29186–29193. https://doi.org/10.1074/jbc.M505000200
Elena C, Carla C, Annalisa M, Samanta T, Youssef S, Marcella P, Alessandra D’a, Caterina S, Giovanni S, Rosario C (2015) S100B-p53 disengagement by pentamidine promotes apoptosis and inhibits cellular migration via aquaporin-4 and metalloproteinase-2 inhibition in C6 glioma cells. Oncology Letters 9(6):2864–2870
Jing L, Qingyuan Y, Wilder Paul T, France C, Weber David J (2010) The calcium-binding protein S100B down-regulates p53 and apoptosis in malignant melanoma. J Biol Chem 285(35):27487–27498
Luis HJ, Laura P, Sheila D, Toni C, Rosa H, Jaume A, Marc M, Francesc M, Maria MJ, Silvia C (2013) Therapeutic targeting of tumor growth and angiogenesis with a novel anti-S100A4 monoclonal antibody. PLoS One 8(9):e72480
Lu Z, Da L, Yi-Ming Z, Yang H, Cheng D, Ma X-X (2016) Effects of receptor for advanced glycation endproducts on microvessel formation in endometrial cancer. BMC Cancer 16(1):93
June C, Koo LM, Kyoung Ho O, Soo KY, Young CH, Kuk BS, Yoon JK, Jeong-Soo W, Hoon LS, Young KS (2011) Interaction effect between the receptor for advanced glycation end products (RAGE) and high-mobility group box-1 (HMGB-1) for the migration of a squamous cell carcinoma cell line. Tumori 97(2):196–202
Lu Xin HJW, Erin P, Xiaoying S, Patricia T, Pingna D, Shan J, Qing C, Spring Denise J, Padmanee S (2017) Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature 543(7647):728–732
Joseph M, Carson William E (2013) Review of S100A9 biology and its role in cancer. BBA-Rev Cancer 1835(1):100–109
Ren X, Shao H, Wei Q, Sun Z, Liu N (2009) Advanced glycation end-products enhance calcification in vascular smooth muscle cells. J Int Med Res 37(3):847–854
Yukiko N, Yuji I, Yuka H, Jun-ichi K, Toshihiko N (2013) Advanced glycation end-products enhance calcification in cultured rat dental pulp cells. J Endod 39(7):873–878
Shevde Lalita A, Samant Rajeev S (2014) Role of osteopontin in the pathophysiology of cancer. Matrix Biol 37:131–141
Yoray S, Yael R, Noam C, Amir B-S, Hila S, Tamar G, Neta E (2015) Tumor-derived osteopontin reprograms normal mammary fibroblasts to promote inflammation and tumor growth in breast cancer. Cancer Res 75(6):963–973
Vijayanirmala S, Muthukumar T, Malathi N, Rajan ST (2015) OPN—revisited. Journal of Clinical and Diagnostic Research: JCDR 9(6):ZE10
Rittling SR, Singh R (2015) Osteopontin in immune-mediated diseases. J Dent Res 94(12):1638–1645
Weber Cynthia E, Kothari Anai N, Wai Philip Y, Li Neill Y, Joseph D, Zapf Matthew AC, Franzen Carrie A, Gupta Gopal N, Clodio O, Andrei Z (2015) Osteopontin mediates an MZF1–TGF-β1-dependent transformation of mesenchymal stem cells into cancer-associated fibroblasts in breast cancer. Oncogene 34(37):4821–4833
Wang X, Chao L, Ma G, Chen L, Tian B, Zang Y, Sun J (2008) Increased expression of osteopontin in patients with triple-negative breast cancer. Eur J Clin Investig 38(6):438–446. https://doi.org/10.1111/j.1365-2362.2008.01956.x
Saleh S, Thompson DE, McConkey J, Murray P, Moorehead RA (2016) Osteopontin regulates proliferation, apoptosis, and migration of murine claudin-low mammary tumor cells. BMC Cancer 16(1):359
Dhanashri T, Asutosh S, Reeti B, Kirti L, Sanjay D, Anupama M, Swapnil K, Suhaschandra D, Kundu Gopal C (2013) Association of osteopontin and cyclooxygenase-2 expression with breast cancer subtypes and their use as potential biomarkers. Oncol Lett 6(6):1559–1564
Bramwell Vivien HC, Tuck Alan B, Chapman Judith-Anne W, Anborgh Pieter H, Postenka Carl O, Waleed A-K, Shepherd Lois E, Lei H, Wilson Carolyn F, Pritchard KI (2014) Assessment of osteopontin in early breast cancer: correlative study in a randomised clinical trial. Breast Cancer Res 16(1):R8
Bramwell Vivien HC, Doig Gordon S, Tuck Alan B, Wilson Sylvia M, Tonkin Katia S, Anna T, Francisco P, Vandenberg Theodore A, Chambers Ann F (2006) Serial plasma osteopontin levels have prognostic value in metastatic breast cancer. Clin Cancer Res 12(11):3337–3343
Abu E-AAM, Luc M, Karel G (2011) Expression of high-mobility groups box-1/receptor for advanced glycation end products/osteopontin/early growth response-1 pathway in proliferative vitreoretinal epiretinal membranes. Mol Vis 17:508
Borthwick Lee A, Mann Derek A (2016) Liver: osteopontin and HMGB1: novel regulators of HSC activation. Nat Rev Gastroenterol Hepatol 13(6):320–322
Elena A, Ge X, Tung-Ming L, Fernando M, Aritz L, Yongke L, Naoto K, Raquel U, Neil T, Antoine Daniel J (2016) Signalling via the osteopontin and high mobility group box-1 axis drives the fibrogenic response to liver injury. In: Gut:gutjnl-2015-310752
Gilkes Daniele M (2016) Implications of hypoxia in breast cancer metastasis to bone. Int J Mol Sci 17(10):1669
Das Riku MGH, Kundu Gopal C (2003) Osteopontin stimulates cell motility and nuclear factor κB-mediated secretion of urokinase type plasminogen activator through phosphatidylinositol 3-kinase/Akt signaling pathways in breast cancer cells. J Biol Chem 278(31):28593–28606
Fernando O-M, Elena S, Eloy P-N, Ariadna P-B, Leire A, José S-P, Aranda Francisco I, Enrique L, Gloria P (2015) Osteopontin regulates VEGFA and ICAM-1 mRNA expression in breast carcinoma. Am J Clin Pathol 143(6):812–822
Monalisa B, Anuradha B, Ramesh B, Goutam C, Priyanka G, Pompom G, Mahadeo G, Smita K, Dhiraj K, Santosh K (2014) Osteopontin as a therapeutic target for cancer. Expert Opin Ther Targets 18(8):883–895
Mansoor A, Kundu Gopal C (2010) Osteopontin selectively regulates p70S6K/mTOR phosphorylation leading to NF-κB dependent AP-1-mediated ICAM-1 expression in breast cancer cells. Mol Cancer 9(1):101
Reeti B, Vinit K, Kirti L, Swapnil K, Kundu Gopal C (2009) Activation of JAK2/STAT3 signaling by osteopontin promotes tumor growth in human breast cancer cells. Carcinogenesis 31(2):192–200
Goutam C, Shalini J, Reeti B, Mansoor A, Priyanka S, Vinit K, Kundu Gopal C (2006) The multifaceted roles of osteopontin in cell signaling, tumor progression and angiogenesis. Curr Mol Med 6(8):819–830
Riku D, Mahabeleshwar Ganapati H, Kundu Gopal C (2004) Osteopontin induces AP-1-mediated secretion of urokinase-type plasminogen activator through c-Src-dependent epidermal growth factor receptor transactivation in breast cancer cells. J Biol Chem 279(12):11051–11064
Antje H, Henri W, Matthias K, Matthias K, Dirk V, Helge T, Matthias B (2010) Effects of osteopontin inhibition on radiosensitivityof MDA-MB-231 breast cancer cells. Radiat Oncol 5(1):82
Nassar D, Blanpain C (2016) Cancer stem cells: basic concepts and therapeutic implications. Annu Rev Pathol 11:47–76. https://doi.org/10.1146/annurev-pathol-012615-044438
Atkinson RL, Yang WT, Rosen DG, Landis MD, Wong H, Lewis MT, Creighton CJ, Sexton KR, Hilsenbeck SG, Sahin AA, Brewster AM, Woodward WA, Chang JC (2013) Cancer stem cell markers are enriched in normal tissue adjacent to triple negative breast cancer and inversely correlated with DNA repair deficiency. Breast Cancer Research BCR 15(5):R77. https://doi.org/10.1186/bcr3471
Stephen P (1889) The distribution of secondary growths in cancer of the breast. Lancet 133(3421):571–573
Mikuła-Pietrasik J, Paweł U, Andrzej T, Krzysztof K (2017) The peritoneal “soil” for a cancerous “seed”: a comprehensive review of the pathogenesis of intraperitoneal cancer metastases. Cell Mol Life Sci:1–17
Thomas K, Emilia W, Sabine H-K, Ande Sudharsana R, Sebastian W, Klaus S-O, Marek L (2008) Cancer stem cell markers in common cancers—therapeutic implications. Trends Mol Med 14(10):450–460
Becker J, Wilting J (2017) WNT signaling, the development of the sympathoadrenal-paraganglionic system and neuroblastoma. Cell Mol Life Sci. https://doi.org/10.1007/s00018-017-2685-8
Yong L, Wang W-M, Xue-Lin Z, He H-Q, Xiao-Lei S, Hong Z, Xu X-F, Liang H, Zhu Z, Lei Z (2016) AGE/RAGE promotes thecalcification of human aortic smooth muscle cells via the Wnt/β-catenin axis. Am J Transl Res 8(11):4644
Ulrike S, Franziska A, Janice S, Ulrike S, Pia H, Wolfgang W, Margit L, Iduna F, Shoemaker Robert H, Schlag Peter M (2011) Intervening in β-catenin signaling by sulindac inhibits S100A4-dependent colon cancer metastasis. Neoplasia 13(2):131–IN138
Ulrike S, Wolfgang W, Dominic S, Mike S, Jutta A, Clara L, Fichtner Iduna SPM, Shoemaker Robert H, Ulrike S (2011) S100A4-induced cell motility and metastasis is restricted by the Wnt/β-catenin pathway inhibitor calcimycin in colon cancer cells. Mol Biol Cell 22(18):3344–3354
Dexing F, David H, Yanhua Z, Yan X, Jill M, Heinz N, Mills Gordon B, Ryuji K, Tony H, Zhimin L (2007) Phosphorylation of β-catenin by AKT promotes β-catenin transcriptional activity. J Biol Chem 282(15):11221–11229
Goo L, Tatiana G, Elizabeth M, Ramanarao D, Pal SA, Brown Jeffrey B, Randal M, Yang G–Y, William RJ, Mark EB (2010) Phosphoinositide 3-kinase signaling mediates β-catenin activation in intestinal epithelial stem and progenitor cells in colitis. Gastroenterology 139(3):869–881. e869
Sho-ichi Y, Takanori M (2010) Advanced glycation end products, oxidative stress and diabetic nephropathy. Oxid Med Cell Longev 3(2):101–108
Serban AI, Stanca L, Geicu OI, Munteanu MC, Costache M, Dinischiotu A (2015) Extracellular matrix is modulated in advanced glycation end products milieu via a RAGE receptor dependent pathway boosted by transforming growth factor-beta1 RAGE. J Diabetes 7(1):114–124. https://doi.org/10.1111/1753-0407.12154
He M, Kubo H, Ishizawa K, Hegab AE, Yamamoto Y, Yamamoto H, Yamaya M (2007) The role of the receptor for advanced glycation end-products in lung fibrosis. Am J Physiol Lung Cell Mol Physiol 293(6):L1427–L1436. https://doi.org/10.1152/ajplung.00075.2007
Xiang H, Yong C (2013) Possible participation of receptor for advanced glycation end products (RAGE) in the origin of cancer stem cells in diabetic patients with colon cancer. Med Hypotheses 80(5):620–623
Jeong-Hun K (2014). Protein kinase C (PKC) isozymes and cancer. New J Sci 2014
Huang S, Ouyang N, Lin L, Chen L, Wu W, Su F, Yao Y, Yao H (2012) HGF-induced PKCzeta activation increases functional CXCR4 expression in human breast cancer cells. PLoS One 7(1):e29124. https://doi.org/10.1371/journal.pone.0029124
Polina G, Alexander K, Nataliya B, Melania T, Isabelle P, Arnon N, Izhar H, Tsvee L (2006) cAMP-induced PKCζ activation increases functional CXCR4 expression on human CD34+ hematopoietic progenitors. Blood 107(3):870–879
Suryatheja A, Abhilasha S, Mohamed EG, Simran B, Marshall Jr Gailen D, Gardner Lauren H, Yoshiko S, ElShamy Wael M (2016) Geminin overexpression-dependent recruitment and crosstalk with mesenchymal stem cells enhance aggressiveness in triple negative breast cancers. Oncotarget 7(15):20869
Rihua Z, Xiaolin P, Huang Zuhu WGF, Guoxin Z (2011) Osteopontin enhances the expression and activity of MMP-2 via the SDF-1/CXCR4 axis in hepatocellular carcinoma cell lines. PLoS One 6(8):e23831
Das Shamik SRS, Shevde Lalita A (2011) Hedgehog signaling induced by breast cancer cells promotes osteoclastogenesis and osteolysis. J Biol Chem 286(11):9612–9622
Matteo P, Paola C (2010) Rac and rho GTPases in cancer cell motility control. Cell Commun Signal 8(1):23
Schiraldi M, Raucci A, Munoz LM, Livoti E, Celona B, Venereau E, Apuzzo T, De Marchis F, Pedotti M, Bachi A, Thelen M, Varani L, Mellado M, Proudfoot A, Bianchi ME, Uguccioni M (2012) HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med 209(3):551–563. https://doi.org/10.1084/jem.20111739
Katrin K, Günter F (2013) RAGE regulation and signaling in inflammation and beyond. J Leukoc Biol 94(1):55–68
Sabine H-K, Suchitra N, Thatchawan T, Manoj M, Alok P, Saeid G, Thomas K (2014) Mechanisms of therapeutic resistance in cancer (stem) cells with emphasis on thyroid cancer cells. Front Endocrinol 5:37
Laura C, Stefania L, Maddalena A, Roberta A, Enrico R, Daniela C, Calogero Raffaele A, Federica C (2013) The noninflammatory role of high mobility group box 1/Toll-like receptor 2 axis in the self-renewal of mammary cancer stem cells. The FASEB Journal 27(12):4731–4744
Aparna J, Bijay D, Steel Jason C (2016) Epithelial-to-mesenchymal plasticity of cancer stem cells: therapeutic targets in hepatocellular carcinoma. J Hematol Oncol 9(1):74
Takanobu T, Tadashi U, Akiko S-S, Masayoshi T (2017) Generation of glyceraldehyde-derived advanced glycation end-products in pancreatic cancer cells and the potential of tumor promotion. World J Gastroenterol 23(27):4910
Masayo M, Hidenori K, Yoshiko O, Tomoaki M, Katsuhito M, Takuhito S, Yohei M, Koka M, Shinya F, Atsushi S (2013) Receptor for advanced glycation end products regulates adipocyte hypertrophy and insulin sensitivity in mice: involvement of Toll-like receptor 2. Diabetes 62(2):478–489
Xiaolong M, Jie Z, Shuying L, Mollianne M, Gonzalez-Angulo AM (2012) A new hypothesis for the cancer mechanism. Cancer Metastasis Rev 31(1–2):247–268
Chen S, Tao M, Zhao L, Zhang X (2017) The association between diabetes/hyperglycemia and the prognosis of cervical cancer patients: a systematic review and meta-analysis. Medicine (Baltimore) 96(40):e7981. https://doi.org/10.1097/md.0000000000007981
Villarreal-Garza C, Shaw-Dulin R, Lara-Medina F, Bacon L, Rivera D, Urzua L, Aguila C, Ramirez-Morales R, Santamaria J, Bargallo E, Mohar A, Herrera LA (2012) Impact of diabetes and hyperglycemia on survival in advanced breast cancer patients. Exp Diabetes Res 2012:732027. https://doi.org/10.1155/2012/732027
Hanahan Douglas WRA (2011) Hallmarks of cancer: the next generation. cell 144(5):646–674
Joseph M, MacKerell Alexander D, Weber David J (2007) A search for inhibitors of S100B, a member of the S100 family of calcium-binding proteins. Mini Rev Med Chem 7(6):609–616
Amlanjyoti D, Shampa M, Piya G, Atanu M, Israr A, Seemana B, Tapashi M, Asit M, Koushik R, Sandeep S (2014) Simultaneous inhibition of key growth pathways in melanoma cells and tumor regression by a designed bidentate constrained helical peptide. Pept Sci 102(4):344–358
Björk P, Björk A, Vogl T, Stenström M, Liberg D, Olsson A, Roth J, Ivars F, Leanderson T (2009) Identification of human S100A9 as a novel target for treatment of autoimmune disease via binding to quinoline-3-carboxamides. PLoS Biol 7(4):e1000097
Pili R, Häggman M, Stadler WM, Gingrich JR, Assikis VJ, Björk A, Nordle Ö, Forsberg G, Carducci MA, Armstrong AJ (2011) Phase II randomized, double-blind, placebo-controlled study of tasquinimod in men with minimally symptomatic metastatic castrate-resistant prostate cancer. J Clin Oncol 29(30):4022–4028
Hong Q, Beatrisa L, Ippei S, Guowei W, Cha Soungchul C, Rao Sheetal S, Jianfei Q, Yared H, Nurieva Roza DKC (2014) Generation of a new therapeutic peptide that depletes myeloid-derived suppressor cells in tumor-bearing mice. Nat Med 20(6):676–681
Limin Z, Haowen J, Gang X, Hui W, Bin G, Jun L, Shanghua M, Rong N, Yan J, Qiang D (2015) Proteins S100A8 and S100A9 are potential biomarkers for renal cell carcinoma in the early stages: results from a proteomic study integrated with bioinformatics analysis. Mol Med Rep 11(6):4093–4100
Filip P, Tomasz R, Yuzhu C, Tatjana C-J (2016) The life and works of S100P-from conception to cancer. Am J Cancer Res 6(2):562
Thiruvengadam A, Vijaya R, Duoli S, Zhenghong P, Pal Ashutosh MDS, Bornmann William G, Logsdon Craig D (2013) Designing and developing S100P inhibitor 5-methyl cromolyn for pancreatic cancer therapy. Mol Cancer Ther 12(5):654–662
Kim CE, Lim SK, Kim JS (2012) In vivo antitumor effect of cromolyn in PEGylated liposomes for pancreatic cancer. J Control Release 157(2):190–195. https://doi.org/10.1016/j.jconrel.2011.09.066
Yuki K, Yoichi T, Tomoe K, Kouji K, Takahiko I, Tomoko H, Ichiaki I, Michiteru Y, Kengo S, Fumio S (2013) Identification and characterization of the direct interaction between methotrexate (MTX) and high-mobility group box 1 (HMGB1) protein. PLoS One 8(5):e63073
Takanori M, Sho-ichi Y, Masayoshi T, Seiji U, Kei F, Seiya O (2010) Nifedipine inhibits advanced glycation end products (AGEs) and their receptor (RAGE) interaction-mediated proximal tubular cell injury via peroxisome proliferator-activated receptor-gamma activation. Biochem Biophys Res Commun 398(2):326–330
Jie C, Xiaoyan L, Jie Z, Yueran Z (2012) Targeting HMGB1 inhibits ovarian cancer growth and metastasis by lentivirus-mediated RNA interference. J Cell Physiol 227(11):3629–3638
Dong Y-D, Cui L, Peng C-H, Cheng D-F, Han B-S, Fang H (2013) Expression and clinical significance of HMGB1 in human liver cancer: knockdown inhibits tumor growth and metastasis in vitro and in vivo. Oncol Rep 29(1):87–94
Huang Z, Zhong Z, Zhang L, Wang X, Xu R, Zhu L, Wang Z, Hu S, Zhao X (2015) Down-regulation of HMGB1 expression by shRNA constructs inhibits the bioactivity of urothelial carcinoma cell lines via the NF-κB pathway. Sci Rep 5
Laura P, Xue J, David L, Sandra P, Sandro J, Forest Kelly H, Saad-Jube Zeyana S, Napolitano A, Pagano I, Negi VS (2017) HMGB1 targeting by ethyl pyruvate suppresses malignant phenotype of human mesothelioma. Oncotarget 8(14):22649
Li ML, Wang XF, Tan ZJ, Dong P, Gu J, Lu JH, Wu XS, Zhang L, Ding QC, Wu WG (2012) Ethyl pyruvate administration suppresses growth and invasion of gallbladder cancer cells via downregulation of HMGB1-RAGE axis. Int J Immunopathol Pharmacol 25(4):955–965
Shen M, Lu J, Dai W, Wang F, Xu L, Chen K, He L, Cheng P, Zhang Y, Wang C (2013) Ethyl pyruvate ameliorates hepatic ischemia-reperfusion injury by inhibiting intrinsic pathway of apoptosis and autophagy. Mediat Inflamm 2013
Du G-J, Zhiyu Z, Xiao-Dong W, Yu C, Tyler C, Chun-Su Y, Wang C-Z (2012) Epigallocatechin Gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients 4(11):1679–1691
Granja A, Pinheiro M, Reis S (2016) Epigallocatechin gallate nanodelivery systems for cancer therapy. Nutrients 8(5):307
Li W, Ashok M, Li J, Yang H, Sama AE, Wang H (2007) A major ingredient of green tea rescues mice from lethal sepsis partly by inhibiting HMGB1. PLoS One 2(11):e1153
Mollica L, De Marchis F, Spitaleri A, Dallacosta C, Pennacchini D, Zamai M, Agresti A, Trisciuoglio L, Musco G, Bianchi ME (2007) Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem Biol 14(4):431–441
Smolarczyk R, Cichoń T, Matuszczak S, Mitrus I, Lesiak M, Kobusińska M, Kamysz W, Jarosz M, Sieroń A, Szalak S (2012) The role of glycyrrhizin, an inhibitor of HMGB1 protein, in anticancer therapy. Archivum Immunologiae Et Therapiae Experimentalis 60(5):391–399
Dhumale SS, Waghela BN, Pathak C (2015) Quercetin protects necrotic insult and promotes apoptosis by attenuating the expression of RAGE and its ligand HMGB1 in human breast adenocarcinoma cells. IUBMB Life 67(5):361–373
Julius A, Hopper W (2017) Inhibition of advanced glycation end-product formation by quercetin and catechin: an alternative therapy for treating diabetic complications. Asian J Pharm Clin Res 10(11):173–176
Tang Y, Chen A (2014) Curcumin eliminates the effect of advanced glycation end-products (AGEs) on the divergent regulation of gene expression of receptors of AGEs by interrupting leptin signaling. Lab Investig 94(5):503–516
Lin J, Tang Y, Kang Q, Feng Y, Chen A (2012) Curcumin inhibits gene expression of receptor for advanced glycation end-products (RAGE) in hepatic stellate cells in vitro by elevating PPARγ activity and attenuating oxidative stress. Br J Pharmacol 166(8):2212–2227
Wang C, Nie H, Li K, Zhang Y-x, Yang F, Li C-b, Wang C-f, Gong Q (2012) Curcumin inhibits HMGB1 releasing and attenuates concanavalin A-induced hepatitis in mice. Eur J Pharmacol 697(1):152–157
Kim D-C, Lee W, Bae J-S (2011) Vascular anti-inflammatory effects of curcumin on HMGB1-mediated responses in vitro. Inflamm Res 60(12):1161–1168
Vinod BS, Antony J, Nair HH, Puliyappadamba VT, Saikia M, Narayanan SS, Bevin A, Anto RJ (2013) Mechanistic evaluation of the signaling events regulating curcumin-mediated chemosensitization of breast cancer cells to 5-fluorouracil. Cell Death Dis 4(2):e505
Hasima N, Aggarwal BB (2012) Cancer-linked targets modulated by curcumin. Int J Biochem Mol Biol 3(4):328
Mizumoto S, Takahashi J, Kazuyuki S (2012) Receptor for advanced glycation end products (RAGE) functions as receptor for specific sulfated glycosaminoglycans, and anti-RAGE antibody or sulfated glycosaminoglycans delivered in vivo inhibit pulmonary metastasis of tumor cells. J Biol Chem 287(23):18985–18994
Bongarzone S, Savickas V, Luzi F, Gee AD (2017) Targeting the receptor for advanced glycation endproducts (RAGE): a medicinal chemistry perspective. J Med Chem
Arumugam T, Ramachandran V, Gomez SB, Schmidt Ann M, Logsdon CD (2012) S100P-derived RAGE antagonistic peptide reduces tumor growth and metastasis. Clin Cancer Res 18(16):4356–4364
Galasko D, Bell J, Mancuso JY, Kupiec JW, Sabbagh MN, van Dyck C, Thomas RG, Aisen PS (2014) Clinical trial of an inhibitor of RAGE-Abeta interactions in Alzheimer disease. Neurology 82(17):1536–1542. https://doi.org/10.1212/wnl.0000000000000364
Nakamura K, Yamagishi S-i, Nakamura Y, Takenaka K, Matsui T, Jinnouchi Y, Imaizumi T (2005) Telmisartan inhibits expression of a receptor for advanced glycation end products (RAGE) in angiotensin-II-exposed endothelial cells and decreases serum levels of soluble RAGE in patients with essential hypertension. Microvasc Res 70(3):137–141
Chen X-F, Zou J-J, Tang W, Lin W-D, Sun L-L, Bao Y, Chen H-Y, Zhang B, Ye F, Liu Z-M (2016) Metformin corrects RAGE overexpression caused signaling dysregulation and NF-κB targeted gene change. Int J Clin Exp Med 9(2):2913–2920
Kumar AA, Hayati ON, Subramani P, Urmila B (2017) Curcumin: the spicy modulator of breast carcinogenesis. J Exp Clin Cancer Res 36(1):98
Zhang G, Xue Y-m, GAO F, Zhu B (2010) AGE-RAGE interaction promotes the proliferation of human colon carcinoma SW-480 cells [J]. Journal of Medical Postgraduates 9:008
Sun YP, Gu JF, Tan X, Bin WCF, Jia XB, Feng L, Liu JP (2016) Curcumin inhibits advanced glycation end product-induced oxidative stress and inflammatory responses in endothelial cell damage via trapping methylglyoxal. Mol Med Rep 13(2):1475–1486
Shen Y, Xu Z, Sheng Z (2017) Ability of resveratrol to inhibit advanced glycation end product formation and carbohydrate-hydrolyzing enzyme activity and to conjugate methylglyoxal. Food Chem 216:153–160
Moridi H, Karimi J, Sheikh N, Goodarzi MT, Saidijam M, Yadegarazari R, Khazaei M, Khodadadi I, Tavilani H, Piri H (2015) Resveratrol-dependent down-regulation of receptor for advanced glycation end-products and oxidative stress in kidney of rats with diabetes. Int J Endocrinol Metab 13(2)
Singh CK, Ndiaye MA, Ahmad N (2015) Resveratrol and cancer: challenges for clinical translation. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 1852(6):1178–1185
Lv L, Shao X, Chen H, Ho C-T, Sang S (2011) Genistein inhibits advanced glycation end product formation by trapping methylglyoxal. Chem Res Toxicol 24(4):579–586
Russo M, Russo GL, Daglia M, Kasi PD, Ravi S, Nabavi SF, Nabavi SM (2016) Understanding genistein in cancer: the “good” and the “bad” effects: a review. Food Chem 196:589–600
Saadat Nadia, Gupta Smiti V (2012). Potential role of garcinol as an anticancer agent. J Oncol 2012
Fumio Y, Toshiaki A, Yoshihiro Y, Hiroyuki N (2000) Antioxidative and anti-glycation activity of garcinol from Garcinia indica fruit rind. J Agric Food Chem 48(2):180–185
Van Puyenbroeck V, Vermeire K (2018) Inhibitors of protein translocation across membranes of the secretory pathway: novel antimicrobial and anticancer agents. Cell Mol life Sci. https://doi.org/10.1007/s00018-017-2743-2
Hsieh HL, Schafer BW, Weigle B, Heizmann CW (2004) S100 protein translocation in response to extracellular S100 is mediated by receptor for advanced glycation endproducts in human endothelial cells. Biochem Biophys Res Commun 316(3):949–959. https://doi.org/10.1016/j.bbrc.2004.02.135
Miyata Y, Nakamoto H, Neckers L (2013) The therapeutic target Hsp90 and cancer hallmarks. Curr Pharm Des 19(3):347–365
Gu H, Yang L, Sun Q, Zhou B, Tang N, Cong R, Zeng Y, Wang B (2008) Gly82Ser polymorphism of the receptor for advanced glycation end products is associated with an increased risk of gastric cancer in a Chinese population. Clin Cancer Res 14(11):3627–3632
Park SJ, Kleffmann T, Hessian PA (2011) The G82S polymorphism promotes glycosylation of the receptor for advanced glycation end products (RAGE) at asparagine 81 comparison of wild-type rage with the g82s polymorphic variant. J Biol Chem 286(24):21384–21392
Wang X, Cui E, Zeng H, Hua F, Wang B, Mao W, Feng X (2012) RAGE genetic polymorphisms are associated with risk, chemotherapy response and prognosis in patients with advanced NSCLC. PLoS One 7(10):–e43734
Pan Hongming, He Lan, Wang Bin, Niu Wenquan. 2014. The relationship between RAGE gene four common polymorphisms and breast cancer risk in northeastern Han Chinese. Sci Rep 4
Qian F, Sun BL, Zhang WY, Ke J, Zhu J (2014) Gly82Ser polymorphism of the receptor for advanced glycation end-product (RAGE) potential high risk in patients with colorectal cancer. Tumor Biol 35(4):3171–3175
Su SC, Hsieh MJ, Chou YE, Fan WL, Yeh CB, Yang SF (2015) Effects of RAGE gene polymorphisms on the risk and progression of hepatocellular carcinoma. Medicine 94(34):e1396
Su S, Chien M, Lin C, Chen M, Yang S (2015) RAGE gene polymorphism and environmental factor in the risk of oral cancer. J Dent Res 94(3):403–411
Xia W, Xu Y, Mao Q, Dong G, Shi R, Wang J, Zheng Y, Xu L, Jiang F (2015) Association of RAGE polymorphisms and cancer risk: a meta-analysis of 27 studies. Med Oncol 32(2):33
Zhao D-C, Lu H-W, Huang Z-H (2015) Association between the receptor for advanced glycation end products gene polymorphisms and cancer risk: a systematic review and meta-analysis. J buon 20:614–624
Liu S, Tong X, He M, Fu X, Zhang Y, Fan H (2016) The receptor for advanced glycation end products gene polymorphisms contribute to cancer susceptibility: evidence from meta-analysis. Int J Clin Exp Med 9(3):5867–5879
Yue L, Zhang Q, He L, Zhang M, Dong J, Zhao D, Ma H, Pan H, Zheng L (2016) Genetic predisposition of six well-defined polymorphisms in HMGB1/RAGE pathway to breast cancer in a large Han Chinese population. J Cell Mol Med 20(10):1966–1973
Taijie L, Weijuan Q, Yanqiong L, Shan L, Xue Q, Zhiming L (2017) Effect of RAGE gene polymorphisms and circulating sRAGE levels on susceptibility to gastric cancer: a case–control study. Cancer Cell Int 17(1):19
Gaens KH, Ferreira I, van der Kallen Carla JH, van Greevenbroek Marleen MJ, Blaak Ellen E, Feskens Edith JM, Dekker Jacqueline M, Giel N, Heine Robert J, ‘t Hart Leen M (2009) Association of polymorphism in the receptor for advanced glycation end products (RAGE) gene with circulating RAGE levels. J Clin Endocrinol Metabol 94(12):5174–5180
Kalea AZ, Schmidt AM, Hudson BI (2009) RAGE: a novel biological and genetic marker for vascular disease. Clin Sci 116(8):621–637
Hofmann MA, Drury S, Hudson BI, Gleason MR, Qu W, Lu Y, Lalla E, Chitnis S, Monteiro J, Stickland MH (2002) RAGE and arthritis: the G82S polymorphism amplifies the inflammatory response. Genes Immun 3(3):123
Tesarova P, Kalousova M, Jachymova M, Mestek O, Petruzelka L, Zima T (2007) Receptor for advanced glycation end products (RAGE)—soluble form (sRAGE) and gene polymorphisms in patients with breast cancer. Cancer Investig 25(8):720–725. https://doi.org/10.1080/07357900701560521
Geroldi D, Falcone C, Minoretti P, Emanuele E, Arra M, D'angelo A (2006) High levels of soluble receptor for advanced glycation end products may be a marker of extreme longevity in humans. J Am Geriatr Soc 54(7):1149–1150
Li S, Li T, Qin W, Qin X, Liu Y, Liu Z (2017) Effect of RAGE gene polymorphisms and circulating sRAGE levels on susceptibility to gastric cancer: a case–control study. Cancer Cell Int 17(1):19
Tomáš K, Marie J, Oto M, Aleš Ž, Tomáš Z, Marta K (2010) Soluble receptor for advanced glycation end-products (sRAGE) and polymorphisms of RAGE and glyoxalase I genes in patients with pancreas cancer. Clin Biochem 43(10–11):882–886
Jing R, Cui M, Wang J, Wang H (2010) Receptor for advanced glycation end products (RAGE) soluble form (sRAGE): a new biomarker for lung cancer. Neoplasma 57(1):55–61
Chen L, Duan Z, Tinker L, Sangi-Haghpeykar H, Strickler H, Ho GY, Gunter MJ, Rohan T, Logsdon C, White DL, Royse K, El-Serag HB, Jiao L (2016) A prospective study of soluble receptor for advanced glycation end-products and colorectal cancer risk in postmenopausal women. Cancer Epidemiol 42:115–123. https://doi.org/10.1016/j.canep.2016.04.004
Moy KA, Jiao L, Freedman ND, Weinstein SJ, Sinha R, Virtamo J, Albanes D, Stolzenberg-Solomon RZ (2013) Soluble receptor for advanced glycation end products and risk of liver cancer. Hepatology (Baltimore, Md) 57(6):2338–2345. https://doi.org/10.1002/hep.26264
Boor P, Celec P, Klenovicsova K, Vlkova B, Szemes T, Minarik G, Turňa J, Šebeková K (2010) Association of biochemical parameters and RAGE gene polymorphisms in healthy infants and their mothers. Clin Chim Acta 411(15–16):1034–1040
Sasahira T, Kirita T, Bhawal UK, Yamamoto K, Ohmori H, Fujii K, Kuniyasu H (2007) Receptor for advanced glycation end products (RAGE) is important in the prediction of recurrence in human oral squamous cell carcinoma. Histopathology 51(2):166–172
Zhao C-B, Bao J-M, Lu Y-J, Zhao T, Zhou X-H, Zheng D-Y, Shan-Chao Z (2014) Co-expression of RAGE and HMGB1 is associated with cancer progression and poor patient outcome of prostate cancer. Am J Cancer Res 4(4):369
Diederichs, Sven, Bulk, Etmar, Steffen, Björn, Ji, Ping, Tickenbrock, Lara, Lang Kerstin, Zänker Kurt S, Metzger, Ralf, Schneider Paul M, Gerke Volker. 2004. S100 family members and trypsinogens are predictors of distant metastasis and survival in early-stage non-small cell lung cancer. Cancer Res 64 (16):5564–5569
Zhang S, Wang Z, Liu W, Lei R, Shan J, Li L, Wang X (2017) Distinct prognostic values of S100 mRNA expression in breast cancer. Sci Rep 7:39786
Gowri Palanissami, Paul Solomon F D. 2017. A novel synergistic pharmaceutical formulation exhibiting anti-cancer activity. Indian Patent 201741034218, 17 Nov 2017. http://ipindia.nic.in/writereaddata/Portal/IPOJournal/1_1556_1/Part-1.pdf
Gruber Lewis S. 2017. Method and composition for treating cancer, killing metastatic cancer cells and preventing cancer metastasis using antibody to advanced glycation end products (AGE). Patent WO 2017143073 A1, 24 Aug 2017. https://patents.google.com/patent/WO2017143073A1/en
Conlan Robert, Gonzalez Deyarina. 2016. Therapeutic agents and use thereof. Patent WO 2016063060 A1, 28 Apr 2016. https://patents.google.com/patent/WO2016063060A1/en
Britten Carolyn. 2017. AGE levels in ER+ metastatic breast cancer patients receiving endocrine therapy. Clinical Trials NCT03092635, 6 Jun 2017. https://clinicaltrials.gov/ct2/show/NCT03092635
Lilly Michael. 2017. Study of pharmacologic manipulation of AGE (advanced glycation end products) levels in prostate cancer patients receiving androgen deprivation therapy. Clinical Trials NCT02946996, 28 Dec 2016. https://clinicaltrials.gov/ct2/show/NCT02946996
Acknowledgements
We would like to thank Prof. B.S. Dwaraknath, Visiting Professor, Central Research facility, Sri Ramachandra Medical College and Research Institute (Deemed to be University) for his valuable suggestions.
Funding
The present review work was supported by the Founder Chancellor Shri.N.P.V Ramasamy Udayar Research fellowship, Sri Ramachandra Medical College and Research Institute (Deemed to be University).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Palanissami, G., Paul, S.F.D. RAGE and Its Ligands: Molecular Interplay Between Glycation, Inflammation, and Hallmarks of Cancer—a Review. HORM CANC 9, 295–325 (2018). https://doi.org/10.1007/s12672-018-0342-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12672-018-0342-9