Abstract
Insulin-like growth factor binding protein-3 (IGFBP-3) is a pro-apoptotic, anti-metastasic, and anti-angiogenic protein. Low serum IGFBP-3 has been associated with risk of more aggressive prostate cancer (PCa). We investigated the impact of nuclear and cytoplasmic IGFBP-3 protein expression levels in PCa by examining their in situ expression across a wide spectrum of primary tumors by immunohistochemical analysis of tissue microarrays. Immunohistochemistry was performed on PCa microarrays constructed from 226 hormone naïve patients who underwent radical prostatectomy. Both cytoplasmic and nuclear IGFBP-3 expressions were scored in a semi-quantitative fashion using an integrated measure of intensity and positivity. The distribution of IGFBP-3 protein expression was examined across the spectrum of epithelial tissues, and its association with standard clinicopathological covariates and tumor recurrence was examined. There was a broad range of IGFBP-3 staining across all histologies examined. Tumor had higher IGFBP-3 cytoplasmic and nuclear staining than benign histologies. For IGFBP-3 nuclear staining, PCa was significantly different than benign prostatic hyperplasia, normal prostate, and prostate intraepithelial neoplasia. As both a continuous and dichotomized variable, higher nuclear IGFBP-3 expression had statistically significant associations with PCa recurrence. The cytoplasmic staining had no significance in any patient subgroup. In patients with low-grade cancer, IGFBP-3 nuclear positivity was a better predictor of recurrence than baseline PSA, tumor margin status, TNM tumor stage, or presence of capsular invasion. High nuclear IGFBP-3 is amongst the strongest predictors of cancer recurrence in patients with low-grade prostate cancers and may therefore play an important role in risk stratification.
Similar content being viewed by others
Introduction
Despite gradual improvements in annual mortality rates, prostate cancer (PCa) remains the leading malignancy in men (excluding basal and squamous cell skin cancers), with over 240,000 new cases estimated for 2011 [22]. Tremendous variability in the clinical behavior of PCa, even within categories defined by traditional clinical and pathologic means of risk stratification, has accelerated interest in the identification of molecular biomarkers of PCa progression. Optimally, early detection and analysis of these biomarkers could accurately stratify patients for appropriate surveillance and risk-adapted therapy. Currently utilized prognostic factors include Gleason grade, tumor stage, and preoperative serum PSA [2, 17, 33]. A recent study identified a Tri-Marker Proliferation Index (proliferation signature genes: MKI67 (Ki-67; also a classic proliferation biomarker), TOP2A (DNA topoisomerase II, alpha), and E2F1 (E2F transcription factor 1)) as a superior prognostic tool in the prediction of PCa recurrence [27]. Clinical use of some of these proliferation markers is already underway in other hormone-responsive malignancies such as breast [7] and gastroentero-pancreatic neuroendocrine tumors [21].
Insulin-like growth factor binding protein (IGFBP)-3 is a potent inducer of apoptosis in PCa (for review see [37]). In addition, anti-angiogenic [26, 29] and anti-metastatic properties have been described in vitro and in vivo [28, 29]. Many of its effects, including inhibition of the NF-κB pathway, appear to be independent of its ability to sequester the IGFs [16]. Epidemiologic studies indicate that higher serum IGF-1 and lower IGFBP-3 levels are independently associated with a greater risk of prostate cancer [3, 4]. Moreover, circulating IGFBP-3 levels were independent predictors of PCa progression in multivariate models that include PSA, biopsy Gleason score, and clinical stage [34].
In a small early study [15], it was reported that IGFBP-3 immunostaining in prostate cancer tissue did not correlate with the clinical outcome of patients undergoing radical prostatectomy. However, a subsequent study performed only in Japanese men demonstrated that intratumoral IGFBP-3 expression in post-neoadjuvant hormonal therapy (NHT) specimens was a useful predictive marker of biochemical recurrence [30]. To further assess the potential clinical significance of IGFBP-3 expression in human PCa, we utilized our large tissue microarray (TMA) platform to examine the association of IGFBP-3 protein-level expression with prostate tissue histology and cancer recurrence. Here, we report for the first time that high nuclear IGFBP-3 is associated with an increased risk of prostate cancer recurrence after radical retropubic prostatectomy.
Methods
Prostate Tissue Microarray Patients
The study cohort consisted of 226 randomly selected, hormone naïve patients who underwent radical retropubic prostatectomy between 1984 and 1995. All prostate tumors were staged according to the 1997 American Joint Committee on Cancer TNM staging system [11] and histologically graded by a single experienced GU pathologist (DS) using the Gleason scoring system [14]. All cases were of the histological type “adenocarcinoma, conventional, not otherwise specified” [38]. Of the 226, 194 were informative for both recurrence outcomes and marker expression data. The median age at the time of surgery was 65 (range 46 to 76). One hundred fourteen (59 %) patients were low grade (Gleason score 2–6); 80 (41 %) were high grade (Gleason score 7–10). Thirty-three patients (17 %) had seminal vesicle invasion (pT3b). Concurrent regional lymphadenectomy accompanied 192 (99 %) cases, of which 10 (5 %) were positive for metastases. One hundred thirty-two (68 %) patients were margin negative and 62 (32 %) were margin positive. Regarding capsular invasion, 43 (22 %) had no invasion, 112 (58 %) had invasion, and 39 (20 %) had extra-capsular extension. In 101 cases (52 %), the tumors were confined to the prostate (organ confined here = T2a or T2b with negative lymph nodes, no capsular extension and with negative surgical margins). Thirty-seven (19 %) patients were considered high risk based on seminal vesicle and/or nodal positivity. The maximum preoperative serum PSA was known for 174 patients (90 %), with a median value of 9.3 ng/ml (range 0.6–96.5). Table 1 shows the clinicopathologic data for this cohort.
A retrospective analysis for outcome assessment was based on detailed, anonymized clinicopathologic information linked to the TMA tissue specimens. Recurrence, defined as a postoperative serum PSA of 0.2 ng/ml or greater, was seen in 67 (35 %) patients. Median follow-up, defined as the time from surgery to recurrence or to last contact in non-recurring patients, was 49.0 months (range 0.1–163) for the entire cohort.
TMA Construction
Formalin-fixed, paraffin-embedded archival tumor specimens were obtained from the UCLA Department of Pathology under IRB approval. Case material was reviewed for tissue array construction by a study pathologist (DS). At least three core tissue biopsies (each 0.6 mm in diameter) were taken from morphologically representative regions of each prostate tumor and precisely arrayed as previously described [23]. Tumor samples were accompanied by matching benign (morphologically normal or hypertrophic) and in situ neoplastic lesions (PIN), when available. For staining, standard non-adhesive sections (5 μm) were transferred to glass slides using an adhesive slide system (Instrumedics Inc., Richmond, IL) to support cohesion of the array elements.
Immunohistochemistry
Immunohistochemical (IHC) staining was performed using a G column-purified hybridoma-derived mouse monoclonal anti-IGFBP3 antibody (R&D Systems MAB305; clone 84728; IgG2b). A standard two-step indirect avidin–biotin complex (ABC) method was used (Vectastain Elite PK-6102, Vector Laboratories, Burlingame, CA) for visualization. Following deparaffinization in xylenes, the array sections were rehydrated in graded alcohols. The sections were placed in 0.01 M sodium citrate buffer (pH 6.0) and heated in a pressure cooker for antigen retrieval. Endogenous peroxidase was quenched with 3 % hydrogen peroxide in methanol at room temperature for 10 min, and then the non-specific protein binding was blocked with 5 % normal goat serum for 60 min. Avidin and biotin were sequentially applied to block endogenous biotin binding sites. The primary antibody was then applied at 10 μg/ml final concentration and incubated at 4 °C overnight. Biotinylated goat anti-mouse IgG was then applied for 60 min at room temperature. The ABC complex was applied for 45 min followed by the chromogen diaminobenzidine (DAB) for 1 min. PBS (10 mM, pH 7.4) was used for all wash steps and dilutions. Incubations were performed in a humidity chamber. The sections were counterstained with hematoxylin, followed by dehydration and mounting. As a negative assay control, non-immune mouse IgG2b antibody (Dako X0944) was applied at the same concentration as the IGFBP-3 antibody.
Scoring of Immunohistochemistry
Semi-quantitative assessment of antibody on the TMAs was performed by a single study pathologist (HY) blinded to the clinicopathological variables. The target tissue for scoring was the glandular prostatic epithelium; scoring of benign tissues did not include basal cells. Tissue spot histology and grading were re-confirmed on the counterstained study slides. IGFBP-3 cytoplasmic expression was scored using two measures, intensity on a 0–3 scale (0 = negative, 1 = weakly positive, 2 = moderately positive, 3 = strongly positive), and percentage of positively stained target cells (range 0–100 % positive) staining at each intensity. To better represent overall protein levels, we combined the frequency and intensity measures into an integrated intensity measure using the following formula: ((percent staining at intensity 3*3) + (percent staining at intensity 2*2) + (percent staining at intensity 1*1))/100. IGFBP-3 nuclear expression was scored noting the percentage of positively stained target cells (range 0–100 % positive). To represent expression within individual cases, the mean pooled integrated intensity (cytoplasmic) and positivity (nuclear) of the invasive tumor spots was used for outcome assessment.
Statistical Analysis
The Kruskal–Wallis and Mann–Whitney U tests were used to determine the significance of IGFBP-3 protein expression differences between nominal clinicopathologic prognostic variables. Associations of IGFBP-3 expression with continuous covariates were tested with the Spearman correlation. We used the Pearson chi-squared test to examine the association of dichotomized IGFBP-3 expression groups versus nominal variables. Recurrence was defined as a rising total prostate-specific antigen (PSA) > 0.2 ng/ml status post prostatectomy, and time to recurrence was calculated from the date of the primary surgery. Patients without recurrence at last follow-up were censored. Kaplan–Meier plots were used to visualize recurrence-free time distributions, and the log–rank test was used to test for differences between them. To assess which covariates associate with recurrence-free time, we fit both univariate and multivariate Cox Proportional Hazards regression models [18]. All P values were two sided and P < 0.05 was considered significant. All statistical analyses were performed using R statistical software (http://www.r-project.org/) and StatView version 5 (SAS Institute Inc., Cary, N.C., USA).
Results
IGFBP-3 Subcellular Localization by Histological Category
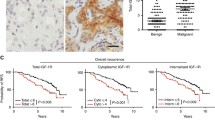
In human prostate tissue, staining of IGFBP-3 was ubiquitous but highly variable across prostate tissues (Fig. 1). Invasive tumor glands are shown displaying negative to weak (Fig. 1a) and moderate to strong (Fig. 1b) diffuse cytoplasmic staining (both cases lacking nuclear staining), whereas a nuclear-predominant staining pattern is seen in the glands of case C (Fig. 1c). While individual cases tend to express a homogenous pattern, rare cases demonstrate distinct regional cytoplasmic or nuclear expression patterns (Fig. 1d; tumor glands in upper and lower tissue spot regions with cytoplasmic- and nuclear-predominant expression, respectively).
Histological staining patterns of IGFBP-3 protein on human prostate tissue microarrays. A variety of immunohistochemical staining patterns for IGFBP-3 protein are shown in a representative sampling of glandular human prostate tissues. The tissue spots are all heavily invested with invasive adenocarcinoma and the protein content is depicted by the brown chromagen deposition. Invasive tumor glands are shown displaying negative to weak (a) and moderate to strong (b) diffuse cytoplasmic staining (both cases lacking nuclear staining), whereas a nuclear-predominant staining pattern is seen in the glands of case c. While individual cases tend to express a homogenous pattern, rare cases demonstrate distinct regional cytoplasmic or nuclear expression patterns (d; tumor glands in upper and lower tissue spot regions with cytoplasmic- and nuclear-predominant expression, respectively) (100× magnification with 400× inserts)
We examined the IGFBP-3 protein expression distribution stratified by histological category (Fig. 2). All 226 hormone naive cases provided epithelium-informative microarray spots; 197 of those included informative spots from cancer tissues. The intensity of IGFBP-3 protein expression in cells staining by immunohistochemistry, as seen in 1,123 informative primary tissue microarray spots containing benign prostatic hyperplasia (BPH; n = 114), morphologically normal prostate (NL; n = 276), prostatic intraepithelial neoplasia (PIN; n = 38) and invasive prostate cancer (PCa; n = 695), are shown as mean bar graphs (Fig. 2a).
IGFBP-3 protein expression stratified by histological category. The distribution of IGFBP-3 protein expression measured by immunohistochemistry and stratified by tissue microarray spot histology is shown in a and b (cytoplasmic intensity [0–3], and nuclear positivity [0–100 %], respectively). The box plot gives the median observation (box dividing line, where visible), the 25th to 75th percentile range (box range), 10th and 90th percentile (whiskers) and <10th and >90th percentile observations (dots). Represented are 114 benign prostatic hyperplasia (BPH), 276 morphologically normal (NL), 38 prostatic intraepithelial neoplasia (PIN), and 695 cancer (PCa) samples, comprising a total of 1,123 informative tissue microarray spots. The mean IGFBP-3 cytoplasmic expression is significantly different comparing all histology group pairs (P < 0.0001, unpaired t test), except PCa versus PIN (P = 0.56). The median nuclear expression in PCa is significantly different versus BPH or NL (both P < 0.0001), and versus PIN (P = 0.0021), and no other comparisons are significant
In general, tumor had higher IGFBP-3 cytoplasmic and nuclear staining than benign histologies. For cytoplasmic staining, all t test comparisons were P < 0.0001 except between PIN and PCa (P = 0.56). For nuclear staining, PCa was significantly different than BPH (P < 0.0001), NL (P < 0.0001), and PIN (P = 0.0021) (Fig. 2b).
There was a broad range of tumor IGFBP-3 cytoplasmic staining in cases which approximated a normal curve (Fig. 3a). However, nuclear staining (0–100 %) was much lower in expression (Fig. 3b) and, when positive, was only occasionally >20 % positive in cells (only seven cases went higher than 50 % pos, max at 70 %). A similar pattern was seen in low- versus high-grade groupings (data not shown). When mean tumor expression (pooled tumor spot data) was examined, a weak but statistically significant inverse correlation between cytoplasmic and nuclear IGFBP-3 expression was found (data not shown). Correlation analysis to cytoplasmic expression was hampered because the nuclear expression component was limited to a small number of cases.
IGFBP-3 protein expression distribution across prostate cancer cases by tissue microarray. The immunohistochemical distribution of IGFBP-3 protein expression is shown for 194 informative primary prostate cancer cases. The relative frequency of expression is stratified by mean cytoplasmic intensity (0–3) and mean nuclear positivity (0–100 %), in a and b, respectively
Nuclear IGFBP-3 Protein Expression is a Strong Predictor of Prostate Cancer Recurrence
We next examined the potential association of IGFBP-3 protein expression with tumor recurrence following radical prostatectomy. Recurrence data was available for 194 IGFBP-3 informative cases (114 low grade and 80 high grade). Case-level expression was derived by pooling the mean integrated intensities of the spots, as previously reported [13]. Supervised survival tree analysis was applied to pooled data, and a dichotomized population was defined with an optimal cutpoint of 10 % mean integrated intensity representing individuals with higher versus lower nuclear IGFBP-3 expression. We examined the association of IGFBP-3 as both a continuous and a dichotomized variable with established prognostic factors, and found that expression of nuclear IGFBP-3 was associated with disease recurrence (Table 2). Figure 4a shows a Kaplan–Meier estimate of cancer recurrence-free time stratified by nuclear IGFBP-3 expression for all cases. Significantly, the median recurrence-free time was 60 months for cases with high nuclear IGFBP-3, and the median was not reached for cases with low nuclear IGFBP-3 (log–rank P = 0.0074). Moreover, in patients with primary low-grade cancer, the presence of nuclear IGFBP-3 was even more predictive of tumor recurrence than in “all cases” (Fig. 4b; log–rank P = 0.0007). In the low-grade group, the median recurrence-free time was 100 months for patients with nuclear IGFBP-3, compared to the median not being reached for patients without nuclear IGFBP-3. Cox proportional hazards analyses were done using established prognostic factors to determine which variables were significant as independent predictors of time to PSA recurrence (Table 2). Of particular note is the strength of nuclear IGFBP-3 predictive power as a dichotomized variable, which remained an independent predictor in multivariate analysis. As an independent predictor, it is close to other conventional clinicopathologic parameters such as stage, especially in primary low-grade cancer.
Kaplan–Meier curves for time to prostate cancer recurrence. Kaplan–Meier curves for time to tumor recurrence stratified by nuclear IGFBP-3 protein expression status are seen in all patients, a (n = 194), and in patients stratified by low tumor grade, (Gleason score range 2–6), b (n = 114). An IGFBP-3 nuclear positivity of ≥9 % and <9 % are considered “High” and “Low” IGFBP-3, respectively. A high nuclear expression phenotype is associated with a higher risk of developing recurrent prostate cancer in both patient cohorts (all patients, P = 0.0074; low-grade cases only, P = 0.0007). Censored times marked by circles and triangles
Cut-point analysis for cytoplasmic staining was attempted (all possible cutpoints in all scoring measures [intensity, positivity and integrated values, each pooled using the mean and median value], in all cases and also the low- and high-grade groups). No statistically significant cutpoint was found. Likewise, cytoplasmic expression used as a continuous value was not statistically significant for any scoring measure or patient strata.
Discussion
Conventional clinicopathological variables serve as important predictors of prostate cancer patient outcomes, but do not elucidate the molecular mechanisms required for cancer cell survival nor suggest therapeutic targets for its treatment. Currently, biopsy Gleason grade together with stage, serum prostate specific antigen (PSA) and tumor volume are used to carefully select men with low risk PCa that will likely benefit from treatment other than radical prostatectomy (RP) such as active surveillance or low dose brachytherapy [1, 8]. However, many men with low grade PCa at biopsy will later be found to have been misclassified, and to later be found to have been diagnosed with high-grade PCa at RP. Therefore, RP Gleason score is better than biopsy Gleason score as an indicator of biochemical recurrence and poor clinical outcome [6, 12, 17, 19], suggesting that Gleason sum upgrading could be clinically relevant to estimate the probability of a more aggressive variant of PCa [10]. A recent study suggests that determination of preoperative IGFBP-3 circulating levels could be useful to predict Gleason score upgrading alone and/or in combination with biopsy T-stage and biopsy Gleason score [36]. These findings correlate well with previous studies showing that circulating IGFBP-3 levels were lowest in patients with bony metastases and lower in patients with metastases to regional lymph nodes than in patients with non-metastatic PCa or in healthy subjects [34]. In addition, local expression of IGFBP-3 has been inversely associated with Gleason score [9].
Tumor suppression by IGFBP-3 in PCa has been characterized in vitro and in vivo, and this suppression can be mediated by IGF-independent mechanisms [5, 35]. Described mechanisms involved in tumor suppression include: induction of senescence [32]; inhibition of angiogenesis [26]; activation of a novel death receptor [20]; internalization via endocytic pathways and nuclear localization [24]; and mitochondrial translocation of RXRα:Nur77 with activation of the intrinsic pathway of apoptosis [25, 31]. We have recently characterized a nuclear export sequence in IGFBP-3 and have demonstrated that mutation of this sequence and sequestration of IGFBP-3 in the nucleus abrogates its apoptosis-inducing properties [31]. This is consistent with our current observation that predominantly nuclear IGFBP-3 staining in PCa tumors (and presumably deficient in ability to undergo IGFBP-3 dependent apoptosis) predict poor outcome as identified by PSA recurrence. We hypothesize that specific post-translational modifications (PTM) and/or protein partners determine nuclear sequestration and inactivation of IGFBP-3 in the progression of PCa. Clearly more studies are needed to elucidate the mechanisms of nuclear sequestration.
An early study pre-TMA methodology assessed IGFBP-3 presence in human PCa in a small number of specimens using standard immunohistochemical (IHC) techniques. Additionally, 24 patients with a preoperative diagnosis of clinically localized prostate adenocarcinoma with 5-year follow-up information were compared with nine normal prostates from organ donors or from patients undergoing cystoprostatectomy. Malignant transformation of prostatic epithelium was associated with a significant decrease in the amount of immunoreactive IGFBP-3; however, this parameter did not correlate with Gleason grade of the tumor or with patient outcome [15]. The presence/absence of predominantly nuclear IGFBP-3, however, was not mentioned in this paper which was also limited by its small sample size.
Similar IHC techniques were used in another study of 42 patients who underwent neoadjuvant hormonal therapy (NHT) and RP [30]. Pre-NHT and post-NHT specimens were examined for expression of IGFBP-3 and apoptosis. Examination of the representative IHC figure reveals that NHT resulted in a significant increase in IGFBP-3 expression in both nuclear and cytoplasmic compartments. This increase in expression correlated with the induction of apoptosis. Patients with high IGFBP-3 expression in the post-NHT specimens had a good prognosis. In these specimens, presumably IGFBP-3 was able to exit the nucleus to induce apoptosis.
Our clinical dataset lacks post-surgical treatment information so that IGFBP-3 expression levels or subcellular localization cannot be linked to future radiotherapy or drug response. Therefore, our study does not directly implicate IGFBP-3 expression levels and sensitivity to subsequent treatment in our cohort and future studies would benefit from inclusion of this variable. In addition, all of the surgical materials are derived from primary prostatectomies. Additional validation studies in patients with hormone refractory recurrence and distant metastases may shed light on IGFBP-3 expression and subcellular localization in these advanced patient groups.
Prostate cancer remains a disease with heterogeneous outcomes, encompassing a wide spectrum of patients from indolent cancer that may not require treatment to patients with highly aggressive cancers for whom treatment is ineffective. Therefore, informative biomarkers are urgently needed to guide both patient surveillance and clinical intervention. This study reports for the first time that the high nuclear expression of IGFBP-3 in primary human prostate cancers is associated with poor outcomes, in both all patients combined, and also for the subset of prostate cancer patients having only low-grade disease. Our study, therefore, identifies nuclear localization of IGFBP-3 (a surrogate of intrinsic pathway activation) as a potential new prognostic marker in PCa and identifies a potential “escape mechanism” (nuclear sequestration) for cancer cell progression. While the value of circulating IGFBP-3 levels remain controversial as a predictive factor for prostate cancer, our study establishes intratumoral IGFBP-3 expression, and specifically subcellular localization, as an important predictive factor for prostate cancer recurrence. As additional controversies exist over the ideal treatment algorithm for low-grade PCa, nuclear sequestration of IGFBP-3 may emerge as a prognostic tool to aid in selecting individual patients for early intervention of PCa.
Abbreviations
- ABC:
-
Avidin–biotin complex
- BPH:
-
Benign prostatic hyperplasia
- H & E:
-
Hematoxylin and eosin
- IGFBP-3:
-
Insulin-like growth factor binding protein-3
- IHC:
-
Immunohistochemistry
- PIN:
-
Prostatic intraepithelial neoplasia
- PCa:
-
Prostate cancer
- PSA:
-
Prostate-specific antigen
- TMA:
-
Tissue microarray
References
Bastian PJ, Carter BH, Bjartell A, Seitz M, Stanislaus P, Montorsi F, Stief CG, Schroder F (2009) Insignificant prostate cancer and active surveillance: from definition to clinical implications. Eur Urol 55(6):1321–1330
Catalona WJ, Smith DS (1998) Cancer recurrence and survival rates after anatomic radical retropubic prostatectomy for prostate cancer: intermediate-term results. J Urol 160(6 Pt 2):2428–2434
Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, Hennekens CH, Pollak M (1998) Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science 279(5350):563–566
Chan JM, Stampfer MJ, Ma J, Gann P, Gaziano JM, Pollak M, Giovannucci E (2002) Insulin-like growth factor-I (IGF-I) and IGF binding protein-3 as predictors of advanced-stage prostate cancer. J Natl Cancer Inst 94(14):1099–1106
Cohen P (2006) Insulin-like growth factor binding protein-3: insulin-like growth factor independence comes of age. Endocrinology 147(5):2109–2111
D'Amico AV, Renshaw AA, Arsenault L, Schultz D, Richie JP (1999) Clinical predictors of upgrading to Gleason grade 4 or 5 disease at radical prostatectomy: potential implications for patient selection for radiation and androgen suppression therapy. Int J Radiat Oncol Biol Phys 45(4):841–846
Dowsett M, Dunbier AK (2008) Emerging biomarkers and new understanding of traditional markers in personalized therapy for breast cancer. Clin Cancer Res: Off J Am Assoc Cancer Res 14(24):8019–8026
Epstein JI (2010) An update of the Gleason grading system. J Urol 183(2):433–440
Figueroa JA, De Raad S, Tadlock L, Speights VO, Rinehart JJ (1998) Differential expression of insulin-like growth factor binding proteins in high versus low Gleason score prostate cancer. J Urol 159(4):1379–1383
Fine SW, Epstein JI (2008) A contemporary study correlating prostate needle biopsy and radical prostatectomy Gleason score. J Urol 179(4):1335–1338, discussion 1338–1339
Fleming ID, Cooper JS, Hensen DE, Hutter RVP, Kennedy BJ, Murphy GP, O’Sullivan B, Sobin LH, Yarbro JW (eds) (1997) American Joint Commitee on Cancer: AJCC Cancer Staging Manual, 5th edn. Lippincott-Raven, Philadelphia, pp 309–312
Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC, Partin AW (2005) Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA: J Am Med Assoc 294(4):433–439. doi:10.1001/jama.294.4.433
Freedland SJ, Seligson DB, Liu AY, Pantuck AJ, Paik SH, Horvath S, Wieder JA et al (2003) Loss of CD10 (neutral endopeptidase) is a frequent and early event in human prostate cancer. Prostate 55(1):71–80
Gleason DF, Mellinger GT (1974) Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol 111(1):58–64
Hampel OZ, Kattan MW, Yang G, Haidacher SJ, Saleh GY, Thompson TC, Wheeler TM, Marcelli M (1998) Quantitative immunohistochemical analysis of insulin-like growth factor binding protein-3 in human prostatic adenocarcinoma: a prognostic study. J Urol 159(6):2220–2225
Han J, Jogie-Brahim S, Harada A, Oh Y (2011) Insulin-like growth factor-binding protein-3 suppresses tumor growth via activation of caspase-dependent apoptosis and cross-talk with NF-kappaB signaling. Cancer Lett 307(2):200–210
Han M, Partin AW, Zahurak M, Piantadosi S, Epstein JI, Walsh PC (2003) Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol 169(2):517–523
Harrell FE (2001) Regression modeling strategies with applications to linear models, logistic regression, and survival analysis. Springer Publishing, New York
Humphrey PA, Frazier HA, Vollmer RT, Paulson DF (1993) Stratification of pathologic features in radical prostatectomy specimens that are predictive of elevated initial postoperative serum prostate-specific antigen levels. Cancer 71(5):1821–1827
Ingermann AR, Yang YF, Han J, Mikami A, Garza AE, Mohanraj L, Fan L et al (2010) Identification of a novel cell death receptor mediating IGFBP-3-induced anti-tumor effects in breast and prostate cancer. J Biol Chem 285(39):30233–30246
Jamali M, Chetty R (2008) Predicting prognosis in gastroentero-pancreatic neuroendocrine tumors: an overview and the value of Ki-67 immunostaining. Endocr Pathol 19(4):282–288
Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA Cancer J Clin 60(5):277–300
Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP (1998) Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 4(7):844–847
Lee KW, Liu B, Ma L, Li H, Bang P, Koeffler HP, Cohen P (2004) Cellular internalization of insulin-like growth factor binding protein-3: distinct endocytic pathways facilitate re-uptake and nuclear localization. J Biol Chem 279(1):469–476
Lee KW, Ma L, Yan X, Liu B, Zhang XK, Cohen P (2005) Rapid apoptosis induction by IGFBP-3 involves an insulin-like growth factor-independent nucleomitochondrial translocation of RXRalpha/Nur77. J Biol Chem 280(17):16942–16948
Liu B, Lee KW, Anzo M, Zhang B, Zi X, Tao Y, Shiry L, Pollak M, Lin S, Cohen P (2007) Insulin-like growth factor-binding protein-3 inhibition of prostate cancer growth involves suppression of angiogenesis. Oncogene 26(12):1811–1819
Malhotra S, Lapointe J, Salari K, Higgins JP, Ferrari M, Montgomery K, van de Rijn M, Brooks JD, Pollack JR (2011) A tri-marker proliferation index predicts biochemical recurrence after surgery for prostate cancer. PLoS One 6(5):e20293
Massoner P, Colleselli D, Matscheski A, Pircher H, Geley S, Jansen Durr P, Klocker H (2009) Novel mechanism of IGF-binding protein-3 action on prostate cancer cells: inhibition of proliferation, adhesion, and motility. Endocr-relat Cancer 16(3):795–808
Mehta HH, Gao Q, Galet C, Paharkova V, Wan J, Said J, Sohn JJ et al (2011) IGFBP-3 is a metastasis suppression gene in prostate cancer. Cancer Res 71(15):5154–5163
Miyata Y, Sakai H, Kanda S, Igawa T, Hayashi T, Kanetake H (2004) Expression of insulin-like growth factor binding protein-3 before and after neoadjuvant hormonal therapy in human prostate cancer tissues: correlation with histopathologic effects and biochemical recurrence. Urology 63(6):1184–1190
Paharkova-Vatchkova V, Lee KW (2010) Nuclear export and mitochondrial and endoplasmic reticulum localization of IGF-binding protein 3 regulate its apoptotic properties. Endocr-relat Cancer 17(2):293–302
Pernicova Z, Slabakova E, Kharaishvili G, Bouchal J, Kral M, Kunicka Z, Machala M, Kozubik A, Souccek K (2011) Androgen depletion induces senescence in prostate cancer cells through down-regulation of Skp2. Neoplasia 13(6):526–536
Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC (1999) Natural history of progression after PSA elevation following radical prostatectomy. JAMA: J Am Med Assoc 281(17):1591–1597
Shariat SF, Lamb DJ, Kattan MW, Nguyen C, Kim J, Beck J, Wheeler TM, Slawin KM (2002) Association of preoperative plasma levels of insulin-like growth factor I and insulin-like growth factor binding proteins-2 and -3 with prostate cancer invasion, progression, and metastasis. J Clin Oncol 20(3):833–841
Silha JV, Sheppard PC, Mishra S, Gui Y, Schwartz J, Dodd JG, Murphy LJ (2006) Insulin-like growth factor (IGF) binding protein-3 attenuates prostate tumor growth by IGF-dependent and IGF-independent mechanisms. Endocrinology 147(5):2112–2121
Terracciano D, Bruzzese D, Ferro M, Mazzarella C, Di Lorenzo G, Altieri V, Mariano A, Macchia V, Di Carlo A (2011) Preoperative insulin-like growth factor-binding protein-3 (IGFBP-3) blood level predicts gleason sum upgrading. The Prostate 2012, 72:100–7
Yamada PM, Lee KW (2009) Perspectives in mammalian IGFBP-3 biology: local vs. systemic action. Am J Physiol Cell Physiol 296(5):C954–C976
Young RH, Srigley JR, Amin MB, Ulbright TM, Cubilla A (2000) Tumors of the prostate gland, seminal vesicle, male urethra, and penis. In Atlas of Tumor Pathology. Washington, DC: Armed Forces Institute of Pathology, pp 210
Acknowledgements
This work was supported in part by P30DK063491, R01AG20954, R01CA100938 (PC); P50CA92131, P01AG034906 (PC and KWL); K12HD34610 and DOD award PC061077 (KWL); and the Jonsson Comprehensive Cancer Center (JCCC) Shared Resource Core Grant at UCLA NIH NCI 2 P30 CA16042-29 (DBS). We thank Vladislava Paharkova for expert technical assistance.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported in part by P30DK063491 R01AG20954, R01CA100938 (PC) P50CA92131 P01AG034906 (PC and KWL) K12HD34610 and DOD award PC061077 (KWL) and the Jonsson Comprehensive Cancer Center (JCCC) Shared Resource Core Grant at UCLA NIH NCI 2 P30 CA16042-29 (DBS).
Rights and permissions
About this article
Cite this article
Seligson, D.B., Yu, H., Tze, S. et al. IGFBP-3 Nuclear Localization Predicts Human Prostate Cancer Recurrence. HORM CANC 4, 12–23 (2013). https://doi.org/10.1007/s12672-012-0124-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12672-012-0124-8