Abstract
Systemic analysis of somatic mutations of other susceptibility genes in syndromic tumors as well as apparently sporadic tumors in well-characterized specimens is lacking. Its clinical relevance has not been studied. Our objective was to determine the frequency of second allele inactivation in syndromic tumors and determine the frequency and potential clinical impact of somatic mutations and loss of heterozygosity (LOH) of the known susceptibility genes in syndromic and sporadic tumors. Nine tumor specimens from clinically characterized VHL mutation, five from SDHB mutation, four from SDHD mutation, two from RET mutation carriers, and eight from apparently sporadic cases were analyzed. Tumor DNA mutation screening of the SDHx, VHL, and RET genes and LOH analyses of the SDHx and VHL genes were performed. The Yates-corrected chi-squared test was used for comparison of the clinical data and the molecular-genetic results. Second allele inactivation in tumors was identified in 83 % of VHL, 80 % of SDHB, and 50 % of SDHD specimen. High prevalence of VHL (6/6, p = 0.024) and SDHB (7/7, p = 0.018) somatic mutations has been identified in the sporadic group compared to all others. In the group of the VHL tumors the SDHB somatic events were significantly lower (2/6; p = 0.045). In 18/19 (95 %) of cases, we were able to demonstrate the presence of at least two concomitant affected susceptibility genes. We conclude that LOH is the most prevalent second allele-inactivating event. SDHB and VHL somatic mutation might play a role in the sporadic forms of tumor development. There is no clinical impact of mutation screening or LOH analysis of tumor specimens.
Similar content being viewed by others
Introduction
Pheochromocytoma is a tumor of the paraganglial system. We use the term pheochromocytoma for adrenal medullary tumors as well as for extra-adrenal retroperitoneal, pelvic, and thoracic paraganglial tumors, because they are usually vasoactive due to release of catecholamines in contrast to head and neck paragangliomas (HNP) [1]. Approximately one out of four pheochromocytoma patients carry a predisposing germline mutation in one of six susceptibility genes. The genes cause distinct clinical syndromes: von Hippel–Lindau disease (VHL; caused by germline mutations in the VHL tumor suppressor gene), multiple endocrine neoplasia type 2 (MEN 2; RET), paraganglioma syndromes type 1 (SDHD), type 3 (SDHC), and type 4 (SDHB) and type 1 neurofibromatosis (NF 1; due to mutations of the NF1 gene) (reviewed by [2]). Recently, germline mutations of the TMEM127, SDHA, and MAX genes have been associated with hereditary pheochromocytoma in rare cases [3–7]. The clinical spectrum varies between the distinct pheochromocytoma syndromes but there is also an evident phenotypic expression difference between patients carrying the same germline defect. The underlying mechanisms are still unclear.
According to Knudson's two-hit model, the inactivation of both alleles of the susceptibility gene is necessary for the development of a tumor in the hereditary setting [8]. So far, for hereditary pheochromocytomas the major and only mechanism identified as a second hit is the loss of the wild type allele (loss of heterozygosity (LOH)). Few studies analyzed tumors for point mutations and promotor region hypermethylation in hereditary pheochromocytoma, but did not identify such somatic changes [9–17]. Further, a significant role of somatic mutations in pheochromocytoma susceptibility genes has not been demonstrated in the development of sporadic pheochromocytoma [13, 15–21].
The aims of our study were: first, to determine the frequency of second allele inactivation in syndromic tumors in terms of point mutations and LOH accordingly to the Knudson two-hit model. Second, we analyzed the tumor material for somatic mutations and LOH of genes being not the susceptibility genes (for example SDHB, SDHC, and SDHD in a patient with a VHL germline mutation). We did not perform the LOH but only point mutation analysis for the RET gene, since RET gene activation is responsible for the tumor development. Third, we determined the frequency and extent of somatic mutations and LOH of pheochromocytoma susceptibility genes in sporadic tumors. Finally we tested, if there was a correlation between the somatic genetic status and clinical setting of pheochromocytoma presentation as a potential tool in the clinical management of the patients.
Materials and Methods
Patients and Tumor Samples
A series of 28 pheochromocytomas from 28 patients from the Freiburg International Pheochromocytoma Registry were used for this study [22]. All patients underwent a detailed clinical and molecular genetic assessment, the latter including germline mutation analysis, in the VHL, RET, SDHB, SDHC, and SDHD genes as previously described [22]. For the purpose of this study, nine tumor specimens from VHL mutation carriers, five from SDHB mutation carriers, four from SDHD mutation carriers, two from RET mutation carriers, and eight tumor specimens from apparently sporadic cases were selected and analyzed.
Tumor DNA Extraction
Using laser-microdissection (PALM Microbeam System, Carl Zeiss MicroImaging GmbH, Jena Germany) tumor cells were collected from formalin-fixed paraffin-embedded tissue and DNA extracted by an automated system (Maxwell 16, Promega, Madison, WI, USA) using the Maxwell 16 FFPE Tissue LEV DNA Purification Kit (Promega) according to manufacturer protocol.
Somatic Point Mutation Analysis
All three exons of the VHL gene, all eight exons of the SDHB gene, all six exons of the SDHC gene, all four exons of the SDHD gene and exons 10, 11, and 13 of the RET gene were amplified by polymerase chain reaction (PCR) using the HotStar Taq DNA polymerase (Qiagen, Hilden, Germany). PCR reaction conditions are available upon request. Every amplicon was analyzed by direct sequencing with the ABI3130 sequencing analyzer according to manufacturer recommendations.
Loss of Heterozygosity Analysis
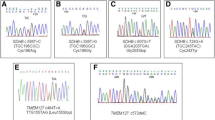
Tumor DNA was extracted as described above. Additionally, DNA from whole-blood leukocytes was extracted using standard procedures. One case with a RET germline mutation was excluded since no blood leukocyte DNA could be obtained. The 27 remaining blood tumor pairs were genotyped for the regions of the VHL gene, the SDHB gene, the SDHC gene, and the SDHD gene using 20 polymorphic microsatellite markers. All the markers were chosen in the close proximity of the studied genes. For primers and the PCR protocols see Electronic Supplementary Material Tables 1 and 2. The PCR products were run on a MegaBACE 500 (GE Healthcare, Pittsburgh, PA, USA) and evaluated with the MegaBACE Genetic Profiler Software Version 2.2 (GE Healthcare). Only polymorphic markers for which the patient has two distinct alleles were possible to determine the presence of an LOH. The latter was defined as a reduction of at least 50 % in peak size in tumor DNA compared to leukocytes DNA (Fig. 1). If the studied polymorphic marker was homozygote in the given patient, it was classified as “non-informative” for the analysis. A gene allele was considered lost if LOH of markers flanking both sides of the gene was identified.
LOH of Marker D3S1335 in patient number 24. On the above panel the analysis of the tumor DNA is shown, one allele with size 148 is represented with corresponding stutter bands, whilst the lower panel represents the germline DNA with both alleles, sizes 148 and 152 with corresponding stutted bands, confirming the loss of allele 152 in the tumor DNA
Statistic Analysis
The Yates-corrected chi-squared test was used for comparison of the clinical data, in particular sex, age at presentation, adrenal or extra-adrenal tumor localization, tumor numbers at presentation, tumor biology (benign or malignant) and patient's molecular characterization (germline mutation screening), and the results of the molecular-genetic analysis in the tumor specimens using the SYSTAT 10 Software. In order to exclude a potential bias of the germline effect in the comparison of somatic mutations and clinical features, we excluded from each analysis the group of tumors with the same germline mutation as the analyzed somatic mutation when present, e.g., we excluded all the VHL tumors when comparing the clinical data and somatic VHL mutations. P values of <0.05 were considered as significant.
Approval and Consent
The study design was approved by the ethical committee of the University Medical Center of Freiburg. All patients signed an informed consent form for participation with the Registry and molecular genetic analysis.
Results
The molecular-genetic characterization and clinical data of the patients as well as the results of the tumor DNA analysis (somatic mutations) are shown in Table 1. If not clearly specified, we use the term somatic mutation for both somatic point mutations as well as presence of LOH. Briefly, 28 tumor specimens from 28 unrelated patients were included in the study. In total, there were 15 male and 13 females patients with age at surgery spanning from 14 to 70 years (median 36.9 years). Regarding the tumor localization, 23 were localized in the adrenal glands and 5 were of extra-adrenal localization, the latter exclusively representing the SDHB mutated tumors. Twelve tumors were multiple and three were malignant.
Considering only the informative cases the inactivation of the second allele among the hereditary tumors could be identified in six of seven VHL-mutated tumors, four of five SDHB-mutated tumors and two of four SDHD-mutated tumors. In the hereditary tumors, no somatic point mutations could be identified as second allele inactivating event. In contrast, one somatic point mutation (SDHD c. 341 A > G) was detected in a sporadic tumor.
The prevalence of somatic mutations (LOH and point mutations) in syndromic and sporadic tumors as well as the comparison of the clinical characteristics and identified somatic mutations is represented in the Tables 2 and 3. Statistically significant were the high prevalence of somatic VHL (six of six informative cases) and SDHB (seven of seven informative cases) mutations in the sporadic form, as well as the low prevalence of the VHL somatic mutations (two of eight informative cases) in the SDHB-mutated tumors. Among the clinical characteristics studied and somatic mutations identified only the presence of a single tumor at presentation was statistically significant associated with the presence of SDHB somatic mutation.
Discussion
Regarding prevalence of second allele inactivation in the hereditary tumors, we were able to identify a LOH in 83 % of VHL informative tumors, 80 % in the SDHB, and 50 % of the informative and total analyzed cases in the SDHD tumor specimens. In all these cases, the loss of the wild-type allele could be confirmed by direct sequencing. Except for the SDHD patients, where we found cases without inactivated second allele, our data are similar to the data published so far. However, the Knudson two hit model is of value for the latter patients is unclear. Whether methylation of the wild-type SDHD allele or inactivation of other genes or regions plays a role in SDHD-associated tumors is still matter of debate, as it is also the particular inheritance pattern in these patients [23, 24].
Only one-point mutation (SDHD c. 341 A > G) could be identified in the tumor DNA. Interestingly, this variant identified in a sporadic patient previously has already been identified in an analysis of sporadic tumors [25]. Further to note is that among the analyzed tumor specimens, a significant high prevalence of VHL (6/6 informative cases; p = 0.024) and SDHB (7/7 informative case; p = 0.018) LOH has been identified in the sporadic group compared to all others. A comparison with so far published data is difficult since in the majority of the published cases the patients were not completely molecular-genetically characterized for the pheochromocytoma-susceptibility genes. However, in more recent studies, the prevalence of SDHB LOH spans from 35–63 %, whilst no recent analysis has been performed for the VHL Locus [16, 17, 19, 20]. If confirmed in larger studies, these results might verify the importance of these genes and gene loci also for the sporadic forms of tumor development. To note is also that compared to all others, in the group of the VHL tumors the SDHB somatic events were significantly lower (2/6 informative cases; p = 0.045), and even if not statistically significant a lower percentage of VHL somatic mutations (1/4, informative cases) was noted in the SDHB group. Whether this somatic event might play a role and correlates with the recently published different transcriptional profiling between the SDHB and VHL pheochromocytoma is unclear, but might become of interest if in the future these pathways will be targeted in therapy strategies, as an easy available and efficient tool in the tumor assessment [26].
Among the clinical data analyzed in our series, the only clinical feature significantly correlating with a somatic mutation was the presence of single tumor at presentation and the SDHB somatic mutation (p = 0.014). Therefore, no clinical implications of somatic mutation testing in the tumor specimens might be recommended (Table 3).
Interestingly, in our series in 18/19 (95 %) of cases we were able to demonstrate the presence of at least two concomitant affected susceptibility genes. A similar finding has been previously described in the MEN2 pheochromocytomas, where a LOH of the VHL gene could be identified in the tumor specimens [27]. However, whether these findings are incidental due to the general chromosomal instability of tumor DNA or are indicating a potential pathogenetic role of different susceptibility genes in the development of hereditary as well as sporadic tumors merits to be clarified in larger genome-wide analysis of tumor specimens.
In summary, in our analysis for LOH and point somatic mutations of the pheochromocytoma susceptibility genes we were able to demonstrate that these genes have an essential role in the hereditary forms of tumor development as well as a potential role in the development of sporadic pheochromocytomas. There is currently no impact on performing these analyses in the routine clinical setting. Further, LOH or point mutations in at least two different susceptibility genes merit further investigations of a possible “multi-hits” mechanism within the known susceptibility genes for the development of hereditary and sporadic pheochromocytomas.
References
Neumann HP, Eng C (2009) The approach to the patient with paraganglioma. J Clin Endocrinol Metab 94(8):2677–2683
Erlic Z, Neumann HP (2009) Familial pheochromocytoma. Hormones (Athens) 8(1):29–38
Qin Y, Yao L, King EE et al (2010) Germline mutations in TMEM127 confer susceptibility to pheochromocytoma. Nat Genet 42(3):229–233
Yao L, Schiavi F, Cascon A et al (2010) Spectrum and prevalence of FP/TMEM127 gene mutations in pheochromocytomas and paragangliomas. JAMA 304(23):2611–2619
Neumann HP, Sullivan M, Winter A et al (2011) Germline mutations of the TMEM127 gene in patients with paraganglioma of head and neck and extraadrenal abdominal sites. J Clin Endocrinol Metab 96(8):E1279–E1282
Comino-Méndez I, Gracia-Aznárez FJ, Schiavi F et al (2011) Exome sequencing identifies MAX mutations as a cause of hereditary pheochromocytoma. Nat Genet 43(7):663–667
Burnichon N, Brière JJ, Libé R et al (2010) SDHA is a tumor suppressor gene causing paraganglioma. Hum Mol Genet 19(15):3011–3020
Knudson AG (1971) Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci USA 68(4):820–823
Eng C, Crossey PA, Mulligan LM et al (1995) Mutations in the RET proto-oncogene and the von Hippel–Lindau disease tumour suppressor gene in sporadic and syndromic phaeochromocytomas. J Med Genet 32(12):934–937
Bender BU, Gutsche M, Gläsker S et al (2000) Differential genetic alterations in von Hippel–Lindau syndrome-associated and sporadic pheochromocytomas. J Clin Endocrinol Metab 85(12):4568–4574
Kytölä S, Nord B, Elder EE et al (2002) Alterations of the SDHD gene locus in midgut carcinoids, Merkel cell carcinomas, pheochromocytomas, and abdominal paragangliomas. Gene Chromosome Canc 34(3):325–332
Dannenberg H, van Nederveen FH, Abbou M et al (2005) Clinical characteristics of pheochromocytoma patients with germline mutations in SDHD. J Clin Oncol 23(9):1894–1901
Korpershoek E, Petri BJ, van Nederveen FH et al (2007) Candidate gene mutation analysis in bilateral adrenal pheochromocytoma and sympathetic paraganglioma. Endocr Relat Cancer 14(2):453–462
Gimenez-Roqueplo AP, Favier J, Rustin P et al (2002) Functional consequences of a SDHB gene mutation in an apparently sporadic pheochromocytoma. J Clin Endocrinol Metab 87(10):4771–4774
Cascon A, Ruiz-Llorente S, Fraga MF et al (2004) Genetic and epigenetic profile of sporadic pheochromocytomas. J Med Genet 41(3):e30
Gimenez-Roqueplo AP, Favier J, Rustin P et al (2003) Mutations in the SDHB gene are associated with extra-adrenal and/or malignant phaeochromocytomas. Cancer Res 63(17):5615–5621
Astuti D, Morris M, Krona C et al (2004) Investigation of the role of SDHB inactivation in sporadic phaeochromocytoma and neuroblastoma. Br J Cancer 91(10):1835–1841
Sun HY, Cui B, Su DW et al (2006) LOH on chromosome 11q, but not SDHD and Men1 mutations was frequently detectable in Chinese patients with pheochromocytoma and paraganglioma. Endocrine 30(3):307–312
Van Nederveen FH, Korpershoek E, Lenders JW et al (2007) Somatic SDHB mutation in an extraadrenal pheochromocytoma. N Engl J Med 357(3):306–308
Benn DE, Croxson MS, Tucker K et al (2003) Novel succinate dehydrogenase subunit B (SDHB) mutations in familial phaeochromocytomas and paragangliomas, but an absence of somatic SDHB mutations in sporadic phaeochromocytomas. Oncogen 22(9):1358–1364
Gimm O, Armanios M, Dziema H et al (2000) Somatic and occult germ-line mutations in SDHD, a mitochondrial complex II gene, in nonfamilial pheochromocytoma. Cancer Res 60(24):6822–6825
Erlic Z, Rybicki L, Peczkowska M et al (2009) Clinical predictors and algorithm for the genetic diagnosis of pheochromocytoma patients. Clin Cancer Res 15(20):6378–6385
Pigny P, Vincent A, Cardot Bauters C et al (2008) Paraganglioma after maternal transmission of a succinate dehydrogenase gene mutation. J Clin Endocrinol Metab 93(5):1609–1615
Neumann HP, Erlic Z (2008) Maternal transmission of symptomatic disease with SDHD mutation: fact or fiction? J Clin Endocrinol Metab 93(5):1573–1575
Braun S, Riemann K, Kupka S et al (2005) Active succinate dehydrogenase (SDH) and lack of SDHD mutations in sporadic paragangliomas. Anticancer Res 25(4):2809–2814
López-Jiménez E, Gómez-López G, Leandro-García LJ et al (2010) Research resource: transcriptional profiling reveals different pseudohypoxic signatures in SDHB and VHL-related pheochromocytomas. Mol Endocrinol 24(12):2382–2391
Koch CA, Huang SC, Zhuang Z et al (2002) Somatic VHL gene deletion and point mutation in MEN 2A-associated pheochromocytoma. Oncogene 21(3):479–482
Acknowledgment
This study has been supported by a grant of the German Cancer Society (Deutsche Krebshilfe); Grant 107995
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Weber, A., Hoffmann, M.M., Neumann, H.P.H. et al. Somatic Mutation Analysis of the SDHB, SDHC, SDHD, and RET Genes in the Clinical Assessment of Sporadic and Hereditary Pheochromocytoma. HORM CANC 3, 187–192 (2012). https://doi.org/10.1007/s12672-012-0113-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12672-012-0113-y