Abstract
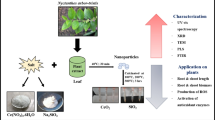
This study aims to green synthesis of sulfur nanoparticles (SNPs) and investigation on its effects at different doses on metabolic profiling of lettuce plants. Whereas previous works only focused on effects of SNPs on growth and stress tolerance of exposed plants, the current work investigates on impact of SNPs on metabolite profiling of plants. SNPs were synthesized via Cinnamomum zeylanicum barks extract and sodium thiosulfate. In order to characterization of SNPs, scanning electron microscopy (SEM), TGA/DTA, and FT-IR techniques were used. The lettuce seeds were pretreated with different concentrations of sulfur nanoparticles (0, 0.001, 0.01, 1, and 10 mg/ml). The leaves of obtained seedlings have been harvested after 6 weeks and subjected for extraction using methanol and then the extracts were analyzed using GC–MS. A MANOVA method, as least squares-discriminant analysis (PLS-DA), was used in order to discriminate between treatment and control groups and changed metabolites have been estimated using HMDB online database. The changed metabolic pathways were estimated using with MetaboAnalyst software, as well. SEM and TEM analyses confirmed highly crystalline pure SNPs with uniform shape and an average particle size range of 43 to 61 nm with average size of 50.33 nm. Results also showed the significant changes in some sugars, amino acids, fatty acids, carboxylic acids, alkanes, and antioxidants levels of lettuce plants treated with different doses of SNPs. PLS-DA analysis indicated that the lettuce group treated with 10 mg/ml of SNP was separated from other groups with different metabolite levels. Some metabolic pathways such as aromatic amino acid biosynthesis, citric acid, and glyoxalate-dicarboxylate pathways exhibited high impact in treated plants than others. It was concluded that the use of C. zeylanicum barks extract can be a rapid and eco-friendly method for SNPs synthesis. On the other hand, although SNPs at high concentration (10 mg/ml) displayed high toxicity on lettuce plants metabolism, but its lower concentration may cause a significant increase in some metabolite levels and made the plants capable to tolerate against environmental stress. Also, applying the appropriate concentration of SNPs (1 mg/ml) can alter metabolic processes and improve the nutritional value of lettuce.









Similar content being viewed by others
Availability of Data and Materials
All data generated from the study and reported in the manuscript are included in the article. Further data sets are available from the corresponding author upon request.
References
Teles, Y. C. F., Horta, C. C. R., Agra, M. F., Siheri, W., Boyd, M., Igoli, J. O., Gray, A. I., Souza, De., & Mde, F. (2018). New sulphated flavonoids from Wissadula periplocifolia (L.) C. Presl (Malvaceae). Molecules, 20, 20161–20172. https://doi.org/10.3390/molecules23112784
Nakai, Y., & Maruyama-Nakashita, A. (2020). Biosynthesis of sulfur-containing small biomolecules in plants. International Journal of Molecular Sciences, 21, 3470. https://doi.org/10.3390/ijms21103470
Brown, L., Scholefield, D., Jewkes, E., Preedy, N., Wadge, K., & Butler, M. (2000). The effect of sulphur application on the efficiency of nitrogen use in two contrasting grassland soils. The Journal of Agricultural Science, 135(2), 131–138.
Bouranis, D. L., Gasparatos, D., Zechmann, B., Bouranis, L. D., & Chorianopoulou, S. N. (2019). The effect of granular commercial fertilizers containing elemental sulfur on wheat yield under Mediterranean conditions. Plants, 8, 2. https://doi.org/10.3390/plants8010002
Bloem, E., Haneklaus, S., & Schnug, E. (2004). Influence of nitrogen and sulphur fertilization on the alliin content of onions and garlic. Journal of Plant Nutrition, 27, 1827–1839.
Pyo, Y. H., Lee, T. C., Logendra, L., & Rosen, R. T. (2004). Antioxidant activity and phenolic compounds of Swiss chard (Beta vulgaris sub species cycla) extracts. Food Chemistry, 85, 19–26.
Salem, N. M., Albanna, L. S., Awwad, A. M., Ibrahim, Q. M., & Abdeen, A. O. (2016). Green synthesis of nano-sized sulfur & its effect on plant growth. Journal of Agricultural Science, 8, 188–194.
Ragab, G. A., & Saad-Allah, K. M. (2020). Green synthesis of sulfur nanoparticles using Ocimum basilicum leaves and its prospective effect on manganese-stressed Helianthus annuus (L.) seedlings. Ecotoxicol Environmental Safety, 191, 110242. https://doi.org/10.1016/j.ecoenv.2020.1102423
Soleimani, M., Aflatouni, F., & Khani, A. (2013). A new and simple method for sulfur nanoparticles synthesis. Colloid Journal, 75, 112–116. https://doi.org/10.1134/S1061933X12060142
Chen, H., Dong, W., Ge, J., Wang, C., Wu, X., Lu, W., & Chen, L. (2013). Ultrafine sulfur nanoparticles in conducting polymer shell as cathode materials for high performance lithium/sulfur batteries. Scientific Reports, 3, 1–6. https://doi.org/10.1038/srep01910
Bura-Nakić, E., Marguš, M., Jurašin, D., Milanović, I., & Ciglenečki-Jušić, I. (2015). Chronoamperometric study of elemental sulphur (S) nanoparticles (NPs) in NaCl water solution: New methodology for SNPs sizing and detection. Geochemical Transactions, 16, 1. https://doi.org/10.1186/s12932-015-0016-2
Chaudhuri, R. G., & Paria, S. (2011). Growth kinetics of sulfur nanoparticles in aqueous surfactant solutions. Journal of Colloid Interface Science, 354, 563–569.
Paralikar, P., & Rai, M. (2017). Bio-inspired synthesis of sulphur nanoparticles using leaf extract of four medicinal plants with special reference to their antibacterial activity. IET Nanobiotechnology, 12(1), 25–31. https://doi.org/10.1049/iet-nbt.2017.0079
Tripathi, R. M., Pragadeeshwara Rao, R., & Tsuzuki, T. (2018). Green synthesis of sulfur nanoparticles and evaluation of their catalytic detoxification of hexavalent chromium in water. RSC Advances, 8, 36345–36352. https://doi.org/10.1039/C8RA07845A
Aliferis, K. A., & Jabaji, S. (2011). Metabolomics—A robust bioanalytical approach for the discovery of the modes-of-action of pesticides: A review. Pesticide Biochemistry and Physiology, 100, 105–117. https://doi.org/10.1016/j.pestbp.2011.03.004
Cowan, D., & Tuffin, M. (2009). Capturing global metabolism. Natural Biotechnology, 27, 1132–1133.
Droux, M. (2004). Sulfur assimilation and the role of sulfur in plant metabolism: A survey. Photosynthesis Research, 79, 331–348.
Gigolashvili, T., & Kopriva, S. (2014). Transporters in plant sulfur metabolism. Frontiers of Plant Science, 5, 1–16. https://doi.org/10.3389/fpls.2014.00442
Hirai, M. Y., Yano, M., Goodenowe, D. B., Kanaya, S., Kimura, T., Awazuhara, M., Arita, M., Fujiwara, T., & Saito, K. (2004). Integration of transcriptomics and metabolomics for understanding of global responses to nutritional stresses in Arabidopsis thaliana. Proceeding of Natural Academic Science of United States of America, 101, 10205–10210.
Krishnakumar, I. M., Issac, A., Johannah, N. M., Ninan, E., Maliakel, B., & Kuttan, R. (2014). Effects of the polyphenol content on the anti-diabetic activity of Cinnamomum zeylanicum extracts. Food & Function Journal, 5, 2208–20. https://doi.org/10.1039/c4fo00130c
Fernie, A. R. (2003). Metabolome characterisation in plant system analysis. Functional Plant Biology, 30, 111–120.
Hong, J., Yang, L., Zhang, D., & Shi, J. (2016). Plant metabolomics: An indispensable system biology tool for plant science. International Journal of Molecular Science, 17, E767. https://doi.org/10.3390/ijms17060767
Yonekura-Sakakibara, K., Tohge, T., Niida, R., & Saito, K. (2007). Identification of a flavonol 7-O-rhamnosyltransferase gene determining flavonoid pattern in Arabidopsis by transcriptome coexpression analysis and reverse genetics. Journal of Biological Chemistry, 282, 14932–14941.
Patra, P., Chudhury, S. R., Mandal, S., Basu, A., Goswami, A., Gogoi, R., Srivastava, C., Kumar, R., & Gopal, M. (2013). Effect sulfur and ZnO nanoparticles on stress physiology and plant (Vignaradiata) nutrition. In: Giri P., Goswami D., Perumal A. (Eds). Advanced nanomaterials and nanotechnology. Springer Proceedings in Physics, vol 143. Springer, Berlin, Heidelberg.
Najafi, S., Razavi, S. M., Khoshkam, M., & Asadi, A. (2020). Effects of green synthesis of sulfur nanoparticles from Cinnamomum zeylanicum barks on physiological and biochemical factors of Lettuce (Lactuca sativa). Physiological Molecular Biology of Plants, 26, 1055–1066. https://doi.org/10.1007/s12298-020-00793-3
Taiz, L., & Zeiger, E. (2013). Plant physiology: Stress physiology, plant physiology (5th ed.). Artmed.
Gil, M. I., Tomas-Barberan, F. A., Hess, P. B., Holcroft, D. M., & Kader, A. A. (2000). Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. Journal of Agriculture Food Chemistry, 48, 4581–4589.
Winkel-Shirley, B. (2002). Biosynthesis of flavonoids and effects of stress. Current Opinion of Plant Biology, 5, 218–223.
Agrawal, S. B., & Rathore, D. (2007). Changes in oxidative stress defense system in wheat (Triticum aestivum L.) and mung bean (Vigna radiate L.) cultivars grown with and without mineral nutrients and irradiated by supplemental ultraviolet-B. Environmental and Experimental Botany, 59, 21–33. https://doi.org/10.1016/j.envexpbot.2005.09.009
Mittler, R., Vanderauwera, S., Gollery, M., & Van Breusegem, F. (2004). Reactive oxygen gene network of plants. Trends of Plant Science, 9, 490–498.
Chuanphongpanich, S., Phanichphant, S., Bhuddasukh, D., Suttajit, M., & Sirithunyalug, B. (2006). Bioactive glucosinolates and antioxidant properties of broccoli seeds cultivated in Thailand. Songklanakarin Journal of Science and Technology, 28, 55–61.
Wedding, R. T., & Black, M. K. (1960). Uptake and metabolism of sulfate by chlorella. I. sulfate accumulation and active sulfate. Plant Physiology, 35, 72–80.
Aires, A., Rosa, E., & Carvalho, R. (2006). Effect of nitrogen and sulfur fertilization on glucosinolates in the leaves and roots of broccoli sprouts (Brassica oleracea var.italica). Journal of the Science of Food and Agriculture, 86, 1512–1516. https://doi.org/10.1002/jsfa.2535
Stewart, J., Chapman, W., Jenkins, G. I., Graham, I., Martin, T., & Crozier, A. (2001). The effect of nitrogen and phosphorus deficiency on flavonol accumulation in plant tissues. Plant, Cell and Environment, 24, 1189–1197.
Nikiforova, V. J., Kopka, J., Tolstikov, V., Fiehn, O., Hopkins, L., Hawkesford, M. J., Hesse, H., & Hoefgen, R. (2005). Systems rebalancing of metabolism in response to sulfur deprivation, as revealed by metabolome analysis of Arabidopsis plants. Plant Physiology, 138, 304–318.
Acknowledgements
The authors thank University of Mohaghegh Ardabili, Ardabil, Iran, for supporting this study.
Funding
None.
Author information
Authors and Affiliations
Contributions
Contribution of authors is defined as the priority of authorship. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
None.
Consent for Publication
Not applicable.
Competing Interests
None.
Research Involving Humans and Animals Statement
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Najafi, S., Razavi, S.M., Khoshkam, M. et al. Green Synthesized of Sulfur Nanoparticles and Its Application on Lettuce Plants Metabolic Profiling. BioNanoSci. 12, 116–127 (2022). https://doi.org/10.1007/s12668-021-00918-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-021-00918-2