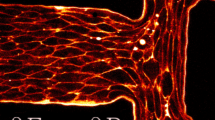
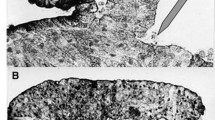
Abstract
This short paper presents a multiscale framework to better understand the mechanisms of biological tissue evolution from molecular to tissue scale. For this, a bottom-up strategy is proposed in which mechano-sensitive molecular processes, the evolution of cell architecture and contraction as well as the interaction between cells and the extra-cellular matrix can be integrated in a single framework. Preliminary studies based on this approach suggest that mechano-sensitive feed-back mechanisms at several length-scales may be a key element to understand tissue adaptivity to tis mechanical environment.




Similar content being viewed by others
References
Baxter, S. C., Morales, M. O., & Goldsmith, E. C. (2008). Adaptive changes in cardiac fibroblast morphology and collagen organization as a result of mechanical environment. Cell Biochemistry and Biophysics, 51(1), 33–44.
Bell, E., Invarsson, B. & Merrill, C. (1979). Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proceedings of the National Academy of Sciences, 76, 1274–1278.
Bischofs, I. B., & Schwarz, U. S. (2003). Cell organization in soft media due to active mechanosensing. Proceedings of the National Academy of Sciences, 100(16), 9274–9279.
Butcher, D. T., Alliston, T., & Weaver, V. M. (2009). A tense situation: Forcing tumour progression. Nature Reviews Cancer, 9, 108–122.
Chrzanowska-Wodnicka, M., & Burridge, K. (1996). Rhostimulated contractility drives the formation of stress fibers and focal adhesions. Journal of Cell Biology, 133(6), 1403–1415.
Costa, K. D., Lee, E. J., & Holmes, J. W. (2003). Creating alignment and anisotropy in engineered heart tissue: Role of boundary conditions in model three-dimensional culture system. Tissue Engineering, 9(4), 567–577.
Dallon, J. C., & Ehrlich, H. P. (2008). A review of fibroblast-populated collagen lattices. Wound Repair and Regeneration, 16, 472–479.
Discher, D. E., Janmey, P., & Wang, Y. (2005). Tissue cells feel and respond to the stiffness of their substrate. Science, 310(5751), 1139–1143.
Dunn, G. A., & Ebendal, T. (1978). Contact guidance on oriented collagen gels. Experimental Cell Research, 111(2), 475–479.
Eastwood, M., Mudera, V. C., McGrouther, D. A., & Brown, R. A. (1998). Effect of precise mechanical loading on fibroblast populated collagen lattices: Morphological changes. Cell Motility and the Cytoskeleton, 40(1), 13–21.
Ehrlich, H. P., Gabbiani, G., & Meda, P. (2000). Cell coupling modulates the contraction of fibroblast-populated collagen lattices. Journal of Cellular Physiology, 184(1), 86–92.
Geiger, B., & Bershadsky, A. (2002). Exploring the neighborhood: Adhesion-coupled cell mechanosensors. Cell, 110(2), 139–142.
Harris, A. K., Stopak, D., & Warner, P. (1984). Generation of spatially periodic patterns by a mechanical instability: A mechanical alternative to the turing model. Journal of Embryology and Experimental Morphology, 80, 1–20.
Harris, A. K., Stopak, D., & Wild, P. (1980). Fibroblast traction as a mechanism for collagen morphogenesis. Nature, 290, 249–251.
Harris, A. K., Wild, P., & Stopak, D. (1980). Silicone rubber substrata: A new wrinkle in the study of cell locomotion. Science, 208(4440), 177–179.
Haston, W. S., Shields, J. M., & Wilkinson, P. C. (1983). The orientation of fibroblasts and neutrophils on elastic substrata. Experimental Cell Research, 146(1), 117–126.
Hill, A. V. (1938). The heat of shortening and the dynamic constant of muscles. Proceedings of the Royal Society of London, 126(843), 136–195.
Huang, S., & Ingber, D. E. (1999). The structural and mechanical complexity of cell-growth control. Nature Cell Biology, 1(5), E131–E138.
Lambert, A., Nusgens, B. V., & Lapiere, M. (1998). Mechano-sensing and mechano-reaction of soft connective tissue cells. Advances in Space Research, 21(8/9), 1081–1091.
Makale, M. (2007). Cellular mechanobiology and cancer metastasis. Birth Defects Research (Part C), 81, 329–343.
Martin, P. (1997). Wound healing: Aiming for perfect skin regeneration. Science, 276, 75–81.
Paszek, M., Zahir, N., Johnson, K., Lakins, J., Rozenberg, G., Gefen, A., et al. (2005). Tensional homeostasis and the malignant phenotype. Cancer Cell, 8, 241–254.
Pelham, R. J., & Wang, Y. L. (1997). Cell locomotion and focal adhesions are regulated by substrate flexibility. Proceedings of the National Academy of Sciences of the United States of America, 94, 13661–13665.
Rome, L. C., Cook, C., Syme, D. A., Connaughton, M. A., Ashley-Ross, M., Klimov, B., et al. (1999). Trading force for speed: Why superfast crossbridge kinetics leads to superlow forces. Proceedings of the National Academy of Sciences, 96, 5826–5831.
Sawhney, R. K., & Howard, J. (2002). Slow local movements of collagen fibers by fibroblasts drive the rapid global self organization of collagen gels. The Journal of Cell Biology, 157(6), 1083–1091.
Semesh, T., Geiger, B., Bershadsky, A. D., & Kozlov, M. (2005). Focal adhesions as mechanosensors: a physical mechanism. Proceedings of the National Academy of Sciences, 102(35), 12383–12388.
Smith, R. C., Cande, W. Z., Craig, R., Tooth, P. J., Scholey, J. M., & Kendrick-Jones, J. (1983). Regulation of myosin filament assembly by light-chain phosphorylation. Philosophical Transactions of the Royal Society of London, 302(1108),73–82.
Solon, J., Levental, I., Sengupta, K., Georges, P. C., & Janmey, P. A. (2007). Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophysical journal, 93(12), 4453–4461.
Sun, D. N., Gu, W. Y., Guo, X. E., Lai, W. M., & Mow, V. C. (2008). A mixed finite element formulation of triphasic mechano-electrochemical theory for charged, hydrated biological soft tissues. International Journal for Numerical Methods in Engineering, 45, 1375–1402.
Tamariz, E., & Grinnell, F. (2002). Modulation of fibroblast morphology and adhesion during collagen matrix remodeling. Molecular Biology of the Cell, 13, 3915–3929.
Vernerey, F. J. (2011). Advances in cell mechanics, on the application of multiphasic theories to the problem of cell-substrate mechanical interactions. New York: Springer.
Vernerey, F. J., & Farsad, M. (2011). A constrained mixture approach to mechano-sensing and force generation in contractile cells. Journal of the Mechanical Behavior of Biomedical Materials. doi:10.1016/j.jmbbm.2011.05.022.
Vernerey, F. J., & Farsad, M. (2011). An eulerian/xfem formulation for the large deformation of cortical cell membrane. Computer Methods in Biomechanics and Biomedical Engineering, 14(5), 433–445.
Weiss, P., & Garber, B. (1952). Shape and movement of mesenchyme cells as functions of the physical structure of the medium contributions to a quantitative morphology. Proceedings of the National Academy of Sciences of the United States of America, 38(3), 264–280.
Weiss, P., & Garber, B. (1952). Shape and movement of mesenchyme cells as functions of the physical struture of the medium contributions to a quantitative morphology. Proceedings of the National Academy of Sciences, 38, 264–280.
Acknowledgement
FJV gratefully acknowledges the University of Colorado CRCW Seed Grant in support of this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vernerey, F.J., Foucard, L. & Farsad, M. Bridging the Scales to Explore Cellular Adaptation and Remodeling. BioNanoSci. 1, 110–115 (2011). https://doi.org/10.1007/s12668-011-0013-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-011-0013-6