Abstract
The contamination risks by naturally derived toxic elements must be assessed to achieve a sustainable geo-environment when utilizing excavated surplus soils. To estimate the controlling factors and risks of groundwater pollution associating with the application of recycled excavated surplus soils, the sequentially extracted fractions of major and toxic elements were analyzed and compared to the results of the simple batch leaching test. The concentrations of bulk B and Pb of the Holocene marine clay layer Ma13 were the maximum 75 ppm and 28 ppm at the middle depth and varied similar to the change of clay fraction, while the bulk As concentration was the maximum 12 ppm at the upper part of the Ma13. The B adsorbed onto the clay minerals was easily desorbed under the neutral pH condition. Arsenic was released especially from the transitional sandy silt layers at the upper and lower parts of Ma13 where contacting with oxic groundwater. The 0.45-µm filter required by Japanese regulations does not efficiently remove colloidal particles resulting in poor reproducibility of batch leaching tests, especially for Pb. Also, relative indices of metal mobility suggest that the long-term risk of groundwater contamination via the reuse of excavated surplus soils will not be accurately estimated only by the simple batch leaching test. The change of redox and pH conditions associating with relocation and preservation must be considered to fully evaluate the risk of toxic element mobilization of the excavated surplus soils.

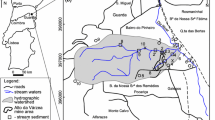
(Modified from Ichihara 1993)





(Recompiled from Ito et al. (2018))


Similar content being viewed by others
References
Akai J, Izumi K, Fukuhara H, Masuda H, Nakano H, Yoshimura T, Ohfuji H, Md Anawar H, Akai K (2004) Mineralogical and geomicrobiological investigations on groundwater arsenic enrichment in Bangladesh. Appl Geochem 19:215–230
Alvarez MB, Domini CE, Garrido M, Lista AG, Fernández-Band BS (2011) Single-step chemical extraction procedures and chemometrics for assessment of heavy metal behaviour in sediment samples from the Bahía Blanca estuary, Argentina. J Soils Sediments 11:657–666
Buatier MD, Peacor DR, O’Neil JR (1992) Smectite-illite transition in Barbados accretionary wedge sediments: TEM and AEM evidence for dissolution/crystallization at low temperature. Clay Clay Miner 40:65–80
Choi W-W, Chen KY (1979) Evaluation of boron removal by adsorption on solids. Environ Sci Technol 13:189–196
Danhara T, Yamashita T, Iwano H, Kasuya M (1992) An improved system for measuring reflective index using the thermal immersion method. Quatern Int 13(14):89–91
Faure G (1998) Principles and applications of geochemistry, 2nd edn. Prentice Hall in Upper Saddle River, New Jersey
Filgueiras AV, Lavilla I, Bendicho C (2002) Chemical sequential extraction for metal partitioning in environmental solid samples. J Environ Monit 4:823–857
Francesconi KA, Edmonds JS (1996) Arsenic and marine organisms. Adv Inorg Chem 44:147–189
Freed RL, Peacor DR (1992) Diagenesis and the formation of authigenic illite-rich I/S crystals in gulf coast shales: TEM study of clay separates. J Sediment Petrol 62:220–234
Geological Survey of Japan (AIST) (2018) Geochemical map of sea and land of Japan. https://gbank.gsj.jp/geochemmap/ocean/data/seadata.htm. Accessed 18 Dec 2018 (in Japanese)
Goldberg S, Glaubig RA (1986) Boron adsorption on California soils. Soil Sci Soc Am J 50:1173–11176
Hass A, Fine P (2010) Sequential selective extraction procedures for the study of heavy metals in soils, sediments, and waste materials: a critical review. Environ Sci Technol 40:365–399
Hattori S, Ohta T, Kikuchi Y (2007) Assessment of judgment standard for acid water drainage from rock muck at the Hakkouda tunnel. J Jpn Soc Eng Geol 47(6):323–336 (in Japanese with English abstract)
Hover VC, Peacor DR (1999) Direct evidence for potassium uptake in smectite during early diagenesis of marine sediments and MORB: balancing the global potassium budget. Clay mineral society meeting program and abstracts, 36th Annual Meeting, Purdue University, West Lafayette, p 50
Ichihara M (ed) (1993) The Osaka Group. Sogen-sha, Osaka. ISBN 4422220039 (in Japanese)
Imoto Y, Yasutaka T, Someya M, Higashino K (2018) Influence of solid–liquid separation method parameters employed in soil leaching tests on apparent metal concentration. Sci Total Environ 624:96–105
Ito K (2012) Soil, clay and minerals on metals contaminated groundwater and soil. Nendokagaku (J Clay Sci Soc Jpn) 50(3):144–153 (in Japanese with English abstract)
Ito H, Masuda H, Kusakabe M (2003) Some factors controlling arsenic concentrations of groundwater in the northern part of Osaka prefecture. J Groundw Hydrol 45(1):3–18 (in Japanese with English abstract)
Ito T, Ito S, Nagata H (2013) Management system on the tunnel goaf with natural heavy metal. J Jpn Soc Civil Eng Ser F4 69(4):I_137–I_144 (in Japanese with English abstract)
Ito H, Masuda H, Oshima A (2018) Concentrations of the naturally-derived toxic elements and its geochemical characteristics of the alluvial marine clay layer of Osaka Plain, Japan. In: Proceedings of the 8th International Congress on Environmental Geotechnics, vol 1, pp 504–511
Iwasaki T, Watanabe T (1998) Distribution of Ca and Na ions in dioctahedral smectites and interstratified dioctahedral mica/smectites. Clay Clay Miner 36(1):73–82
Japanese Geotechnical Society (2015) JGS 1221–2012 method for obtaining soil samples using thin-walled tube sampler with fixed piston. Japanese geotechnical society standards: geotechnical and geo-environmental investigation methods, 1st edn. Maruzen Publishing Co., Ltd., Tokyo. ISBN 978-4-88644-820-0
Japanese Industrial Standard Association (2009a) JIS A 1204 test method for particle size distribution of soils. http://www.jisc.go.jp/app/jis/general/GnrJISSearch.html. Accessed 4 Jan 2019 (in Japanese)
Japanese Industrial Standard Association (2009b) JIS A 1203 test method for water content of soils. http://www.jisc.go.jp/app/jis/general/GnrJISSearch.html. Accessed 4 Jan 2019 (in Japanese)
Japanese Industrial Standard Association (2009c) JIS A 1226 test method for ignition loss of soils. http://www.jisc.go.jp/app/jis/general/GnrJISSearch.html. Accessed 4 Jan 2019 (in Japanese)
Japanese Ministry of the Environment (1991) Environmental agency notifications no. 46 (environmental standards relating to contamination of soil). http://www.env.go.jp/kijun/dojou.html. Accessed 25 Feb 2019 (in Japanese)
Japanese Ministry of the Environment (2003a) Environmental agency notifications no. 18 (analytical methods of simple batch leaching test). https://www.env.go.jp/water/dojo/law/kokuji.html. Accessed 25 Feb 2019 (in Japanese)
Japanese Ministry of the Environment (2003b) Environmental agency notifications no. 19 (analytical methods of acid leaching test). https://www.env.go.jp/water/dojo/law/kokuji.html. Accessed 25 Feb 2019 (in Japanese)
Japanese Ministry of the Environment (2017a) Amendment to the soil contamination countermeasures act. http://elaws.e-gov.go.jp/search/elawsSearch/elaws_search/lsg0500/detail?lawId=414AC0000000053. Accessed 25 Feb 2019 (in Japanese)
Japanese Ministry of the Environment (2017b) Enforcement notice of the soil contamination countermeasures act, no. 1703313. https://www.env.go.jp/water/dojo/law/kaisei2009/no_100305002.pdf. Accessed 25 Feb 2019 (in Japanese)
Japanese Ministry of the Environment (2017c) Enforcement regulation of the soil contamination countermeasures act, ordinance no. 29 of ministry of the environment. http://elaws.e-gov.go.jp/search/elawsSearch/elaws_search/lsg0500/detail?lawId=414M60001000029. Accessed 25 Feb 2019 (in Japanese)
Kabala C, Singh BR (2001) Fractionation and mobility of copper, lead, zinc in soil profiles in the vicinity of a copper smelter. J Environ Qual 30:485–492
Kanjo Y, Nouri M, Kase T (2008) Application of modified BCR sequential extraction procedure for evaluation of heavy metal leaching in contaminated soil. J Jpn Soc Civil Eng G64(4):304–313 (in Japanese with English abstract)
Kansai Geo-informatics Network (KG-NET) (2007) Shi-Kansai Jiban-from Osaka Plain to Osaka Bay area. KG-NET (in Japanese)
Kohn MJ, Riciputi LR, Stakes D, Orange DL (1998) Sulfur isotope variability in biogenic pyrite: reflections of heterogenous bacterial colonization? Am Miner 83:1454–1468
Leśniewska B, Kisielewska K, Wiater J, Godlewska-Żyłkiewicz B (2016) Fast and simple procedure for fractionation of zinc in soil using an ultrasound probe and FAAS detection. Validation of the analytical method and evaluation of the uncertainty budget. Environ Monit Assess 188:29. https://doi.org/10.1007/s10661-015-5020-6
Machida H, Arai F (2003) Atlas of tephra in and around Japan. University of Tokyo Press, Tokyo (in Japanese). ISBN 4-13-060745-6
Masuda H (2018) Arsenic cycling in the earth’s crust and hydrosphere: interaction between naturally occurring arsenic and human activities. Prog Earth Planet Sci 5:68
Masuda H, Peacor DR, Dong H (2001) Transmission electron microscopy study of conversion of smectite to illite in mudstones of the Nankai trough: contrast with coeval bentonites. Clay Clay Miner 49(2):109–118
Masuda F, Irizuki T, Fujiwara O, Miyahara B, Yoshikawa S (2002) A Holocene sea-level curve constructed from a single core at Osaka, Japan (a preliminary note). Ser Geol Miner 59(1):1–8
Matschullat J (2000) Arsenic in geosphere: a review. Sci Total Environ 249:297–312
McGarvey GB, Ross KJ, McDougall TE, Turner CW (1999) Lead corrosion and transport in simulated secondary feedwater. Atomic energy of Canada limited. https://inis.iaea.org/collection/NCLCollectionStore/_Public/31/030/31030413.pdf. Accessed 9 Jan 2019 (ISSN 0067-0367)
Mitamura M, Masuda H (1998) Arsenic content of drilling cores in Osaka and its surrounding area. In: Proceedings of the 8th Symposium on Geo-Environments and Geo-Technics, pp 85–88 (in Japanese with English abstract)
Mozer A (2010) Authigenic pyrite framboids in sedimentary facies of the Mount Wawel formation (eocene), King George Island, west Antarctica. Pol Polar Res 31(3):255–272
Neff JM (2002) Chapter 3: arsenic in the ocean. In: Bioaccumulation in marine organisms: effect of contaminants from oil well produced water. Elsevier Science, pp 57–78. ISBN 9780080527840
Nishizawa T, Kato M, Kitazawa H, Sato T (2012) Chemical speciation and release of arsenic to groundwater in Seino Basins, Nobi Plain. J Jpn Soc Civil Eng Ser C 68(4):670–679 (in Japanese with English abstract)
Nixon SW (1988) Physical energy inputs and the comparative ecology of lake and marine ecosystems. Limnol Oceanogr 33:1005–1025
Ogawa Y, Masuda S, Shinoda K (2014) Arsenic dissolution from waste dumps containing marine sediment. J Geogr 123(6):936–948 (in Japanese with English abstract)
Okumura K, Sakurai K, Nakamura N, Morimoto Y (2007) Environmental impacts of naturally-occurring heavy metals and countermeasures. J Geogr 116(6):892–905 (in Japanese with English abstract)
Osaka Prefecture Government (2018) Results of sea bottom sediments investigation in Osaka Bay, 2012. http://www.pref.osaka.lg.jp/kankyohozen/osaka-wan/teisitsu.html. Accessed 18 Dec 2018 (in Japanese)
Osawa S, Amita M, Yamada M, Mishima T, Kazahaya K (2010) Geochemical features and genetic process of hot-spring waters discharged from deep hot-spring wells in the Miyazaki Plain, Kyushu island, Japan: diagenetic dehydrated fluid as a source fluid of hot-spring water. J Hot Spring Sci 59:295–319 (in Japanese with English abstract)
Popa R, Kinkle BK, Badescu A (2004) Pyrite framboids as biomarkers for iron-sulfur systems. Geomicrobiol J 21:193–206
Public Works Research Institute et al (2015) Handbook of excavated surplus soils containing naturally-derived heavy metals. Taisei Shuppan, Tokyo (in Japanese). ISBN 978-4-8028-3193-2
Rauret G, López-Sánchez JF, Sahuquillo A, Rubio R, Davidson C, Ure A, Quevauvillerc P (1999) Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J Environ Monit 1(1):57–61
Sahuquillo A, Rigol A, Rauret G (2003) Overview of the use of leaching/extraction tests for risk assessment of trace metals in contaminated soils and sediments. Trends Anal Chem 22:152–159
Savage KS, Tingle TN, O’Day PA, Waychunas GA, Bird DK (2000) Arsenic speciation in pyrite and secondary weathering phases, Mother Lode gold district, Tuolumne County, California. Appl Geochem 15:1219–1244
Smedley PL, Kinniburg DG (2002) A review of the source, behavior and distribution of arsenic in natural waters. Appl Geochem 17:517–568
Sudo K, Yoneda T, Ogawa Y, Yamada R, Inoue C, Tsuchiya N (2010) Effect of weathering on changes of leaching properties and chemical forms of heavy metals in a sedimentary rock of Tatsunokuchi formation. J Jpn Soc Eng Geol 51(4):181–190 (in Japanese with English abstract)
Sutherland RA (2010) BCR®-701: a review of 10 years of sequential extraction analyses. Anal Chim Acta 680(1–2):10–20
Takahashi R, Kakihara Y, Hara J, Komai T (2011) Leaching process of arsenic from sedimentary rocks and change in leaching amount with weathering: examples from the Miocene Kawabata and Karumai formations, central Hokkaido, Japan. J Geol Soc Jpn 117(10):565–578 (in Japanese with English abstract)
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51(7):844–851
Thomas RP, Ure AM, Davison CM, Littlejohn D, Rauret G, Rubio R, Lopez-Sanches JF (1994) Three-stage sequential extraction procedure for the determination of metals in river sediments. Anal Chem Acta 286:423–429
Ure AM, Quevauviller P, Muntau H, Griepink B (1993) Speciation of heavy metals in soils and sediments. An account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the Commission of the European Communities. Int J Environ Anal Chem 51:135–151
Vail PR, Audemard F, Boeman SA, Eisner PN, Perez-Cruz C (1991) The stratigraphic signatures of tectonics, eustasy and sedimentology: an overview. In: Einsele G, Ricken W, Seilacher A (eds) Cycles and events in stratigraphy, 6th edn. Springer-Verlag, Berlin, pp 617–659
Wada S (2008) Clays and soil pollution. Nendokagaku (J Clay Sci Soc Jpn) 47(3):185–195 (in Japanese)
Wang N, Ouyang T, Yun SJ, Iwashima K (1997) The chemical forms of As in soils of Chiba prefecture. Kankyo to Sokuteigijutu (Environ Meas Tech) 24:7–11 (in Japanese)
Williams LB, Hervig RL (2005) Lithium and boron isotopes in illite-smectite: the importance of crystal size. Geochim Cosmochim Acta 69:5705–5716
Williams LB, Hervig RL, Holloway JR, Hutcheon I (2001) Boron isotope geochemistry during diagenesis: part 1. Experimental determination of fractionation during illitization of smectite. Geochim Cosmochim Acta 65:1769–1782
Williams LB, Turner A, Hervig RL (2007) Intercrystalline boron isotope partitioning in illite-smectite: testing the geothermometer. Am Miner 92:1958–1965
Yamaguchi H, Montani S (2002) Biological production in coastal seas with relation to benthic organisms. Jpn J Limnol 63:241–248 (in Japanese with English abstract)
Yasuhara M, Irizuki T, Yoshikawa S, Nanayama F (2002) Holocene sea-level changes in Osaka Bay, western Japan: ostracode evidence in a drilling core from the southern Osaka Plain. J Geol Soc Jpn 108(10):633–643
Yasutaka T, Imoto Y, Kurosawa A, Someya M, Higashino K, Kalbe U, Sakanakura H (2017) Effects of colloidal particles on the results and reproducibility of batch leaching tests for heavy metal-contaminated soil. Soils Found 57:861–871
Zimmerman AJ, Weindorf DC (2010) Heavy metal and trace metal analysis in soil by sequential extraction: a review of procedures. Int J Anal Chem 2010:1–7. https://doi.org/10.1155/2010/387803
Acknowledgements
The authors thank N. Kitada and T. Fujiwara for sampling, and K. Okazaki for analytical assistance in the laboratory.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ito, H., Masuda, H. & Oshima, A. Leaching characteristics of naturally derived toxic elements in the alluvial marine clay layer beneath Osaka Plain, Japan: implications for the reuse of excavated soils. Environ Earth Sci 78, 589 (2019). https://doi.org/10.1007/s12665-019-8595-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-019-8595-3