Abstract
An alarming situation of exceedingly high Fe and Mn concentrations in both surface water and groundwater has been identified in a tectonically active region located in Assam-Arakan basin of northeast India. Cross plots, Pearson correlation, principal component analysis and flow net methods were applied to elucidate the present state and possible sources of Fe and Mn contamination. Out of 32 water samples collected for the study, all of them found exceeding 28 times more for Fe and 136 times more for Mn than EPA limit. Correlation and cross plots of pH, TDS, EC, Fe, Mn, Cu, Zn suggest the reduction of both Fe-hydroxides and Mn-oxides in redox condition into dissolve states leading to Fe and Mn elevations in groundwater. Principal component analysis exhibits three PC factors in which PC1 and PC3 reflected oxidized condition and PC2 reflected reducing environment that controlled Fe and Mn concentrations. The study also revealed that source of high Fe and Mn contaminations was controlled by groundwater flows. Water table contour flow net suggests that the Chathe river acts as influent stream and supplies elevated Fe and Mn water into the shallow aquifers. Baseflow from the discharge areas into low lying areas causes accretion of Fe and Mn in pond, oxbow lake, backswamp and shallow aquifers.
Similar content being viewed by others
Introduction
Though human body requires metals like Mn, Fe, Cu, Pb, Zn, As for its metabolism, their high concentrations in domestic and irrigation waters are problematic for reasons of aesthetics, practicality, or because of their impact on health or agricultural sustainability (McArthur et al. 2012). Several researches across the globe have studied elevated trace metals in domestic water from different geological terrains (Sparrow and Uren 1987; Nriagu and Pacyna 1988; Edmunds et al. 1992; Khan et al. 1996; Kraft et al. 1999; Pawar and Nikumbh 1999; Romic and Romic 2003; Buschmann et al. 2007; Ljung and Vahter 2007; Weng et al. 2007; Sanders et al. 2012). In case of Fe, it can occur in two forms, as Fe(II) and as Fe(III). Although Fe(II) is very soluble, Fe(III) does not dissolve appreciably. The extent to which Fe dissolved in groundwater depends on the amount of oxygen in the water and to a lesser extent, upon its degree of acidity, i.e., its pH (Hem 1989; Brown et al. 1999). Generally, when levels of dissolved oxygen in groundwater are greater than 2 mg/l, iron occurs as Fe(III), while at lower dissolved oxygen levels, the iron occurs as Fe(II). High concentration of Fe in drinking water is considered harmful to human health over a long time (Liap 1992; Subba Rao 1993; Subba Rao and Madhusudhana Reddy 2006). Fe concentration up to 2–3 mg/l in drinking water might pose a problem for individuals who suffer Fe storage disease called hemochromatosis (WHO 2005). Mn occurs in two states in the environment, Mn(II) which is widespread and soluble and Mn(IV) which is more insoluble. Acidic, wet, anaerobic and organic soil conditions favour the reduction of Mn(IV) to Mn(II) (Jenne 1968; Gilkes and McKenzie 1988). Mn, in general, precipitates as redox-sensitive and poorly crystalline oxides in soil which can be mobilized or strongly sorbs metal contaminants like Cr, Co, Ni (Suarez and Langmuir 1976; Manceau et al. 1992; Post 1999) and can lead to Mn toxicity in plants (Horsley et al. 2000; Alam et al. 2006; Kogelmann and Sharpe 2006). Mn, its ingestion may adversely affect human health (Kondakis et al. 1989; Wasserman et al. 2006; Spangler and Spangler 2009) with the young and unborn being most at risk (Hafeman et al. 2007; Wasserman et al. 2011; Bouchard et al. 2007, 2011). Such metal concentrations in groundwater have fascinated few researchers in the Assam foreland basin particularly in the Brahmaputra flood plain (Borah et al. 2009; Buragohain et al. 2010; Chakrabarty and Sarma 2011; Haloi and Sarma 2012). However, such study around tectonically active particularly the Naga Thrust where the Assam Shelf buried underneath the Naga Schuppen Belt at the Indo-Myanmar Mobile Belt has not been attempted. The present study attempts to elucidate the state of trace metal concentrations and their possible sources of contaminants particularly Fe and Mn in the study area.
Study area
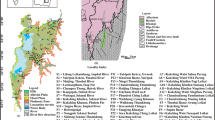
The study area lies between 25°40′ and 25°58′N latitudes and 93°49′ and 93°41′E longitudes and covers part of Dimapur district. The Dimapur town is the largest conurbation of Nagaland state and the 115th most populous city in India. This ever sprawling commercial hub relies primarily on groundwater for various purposes. The Quaternary alluvium is the principal aquifers that act as the groundwater source for domestic uses for these locale residences. The Dhansiri river and the Chathe river which are the tributaries of the Brahmaputra river, originate from Naga Hills and pierce through the Naga Thrust and flow northerly through the study area before joining the Brahmaputra river in its left bank. The study area lies within the Dhansiri valley which is a part of the upper the Assam Shelf and spreads between the Mikir Hills in the west and the Naga foothills in the east (Fig. 1). Geologically the study area is located at the western margin of the Indo-Myanmar Mobile Belt where the Assam Shelf is buried underneath the Naga Schuppen Belt. The line of contact between the Assam Shelf and the Naga Schuppen Belt is said to have been located on the transitional crust (Mathur and Evans 1964; Bhandari et al. 1973). Tectonically, the Assam-Arakan basin comprises the Assam Shelf, the Naga Schuppen Belt and the Assam-Arakan Fold Belt. This category-I sedimentary basin has been defined as a poly-history basin synchronous with the other eastern coast basins of India, concomitant with the rifting and subsequent drifting of the Indian plate from the eastern Gondwanaland (Mathur and Evans 1964). The basin is known for its large hydrocarbon deposits (Corps 1949; Mathur and Evans 1964; Saikia and Dutta 1980; Handique and Bharali 1981; Krishna Rao and Parasad 1982; Raju and Mathur 1995; Kent et al. 2002) where well known oil fields like the Nahorkatia, the Moran and the Rudrasagar are located. The Assam Shelf has large extent of area and belongs to the northeastern prolongation of the Indian Peninsular Shield, bounded in the northwest by the Eastern Himalayan Fold Belt and in the southeast by the Naga-Patkai Fold Belt. The Naga Schuppen Belt is a narrow, elongated complex zone of imbricate thrusts having about 20–35 km width, extending for about 200 km in the hill ranges aligned roughly NNE-SSW direction in the western Nagaland. This zone is strongly imbricated and consists of six to eight major overlapping thrust sheets. The northwestward margin of this belt is conventionally called the Naga Thrust (Mathur and Evans 1964) and is the youngest thrust which separates the foreland and the imbricate thrust zone (Kent et al. 2002). The Assam-Arakan Fold Belt, located in the central part of Naga hills, is considered as a geosynclinal component (Mathur and Evans 1964) bounded between two prominent thrusts viz., the Disang Thrust towards West and the Tapu Thrust to the East (Jaiswal 2009).
Materials and method
In the present study, 32 water samples of pre-monsoon season in the year 2015 were collected. Out of these, each sample from oxbow lake, pond, backswamp, 4 samples from rivers, 3 groundwater samples from depth of ~185 m bgl (below ground level) and 22 shallow groundwater samples from depth ~4 m bgl were collected. The pH, TDS and EC were measured in the field by the field kit, Model No. HI 9811-5, Hanna at the time of sampling. The sampling bottles were thoroughly rinsed using the water to be sampled. In the case of bore wells, the water samples were collected after pumping the water at least for 5 minutes. In the case of open wells, water samples were collected 30 cm below the water level. Samples collected were filtered using 0.45 mm millipore filter paper and acidified with nitric acid (Ultra pure, Merck) for trace metal analyses. The concentrations of Fe, Mn, Cu, Pb, As and Zn ions were measured in triplicate using an inductively coupled atomic emission spectrometer (ICP-AES, Model, ARCOS from Spectro, Germany) at Sophisticated Analytical Instrument Facility (SAIF), Indian Institute of Technology (IIT), Bombay. The trace metals were concentrated from 500 ml solution to 50 ml with warming at 40 °C (APHA 2005). The elevation and depth to water table from below ground level were measured using GPS, Model 60CSX, Garmin and water table measurement kit in 36 open dugwells. These data were used to prepare the water table elevation contour map using triangulation method for pre-monsoon period. For statistical analysis like Pearson correlation, principal component analysis, and cross plots, IBM, SPSS Statistics, Version 20 software was used.
Results
The result of the physiochemical properties and heavy metal concentrations has been shown in Table 1. The water samples have an average pH value of 6.2. The acidic water (pH = 6.1) has been identified in a pond located in the Dhansiri floodplain at Naharbari area. In groundwater, 14 out of 25 samples are acidic in nature (pH ranged from 4.1 to 6.5) where highly acidic water (pH 4.1) is located along the Chathe floodplain at Kraha village. Temperature ranged from 18 ºC to 26 ºC and 19 ºC to 27 °C for surface water and groundwater, respectively, indicating natural condition. TDS of surface water varied from 40 mg/l to 100 mg/l while TDS for groundwater varied from 20 mg/l to 320 mg/l indicating fresh water type (Davis and Dewiest 1966). Similarly, EC values varied from 90 µS/cm to 120 µS/cm in surface water and from 50 µS/cm to 650 µS/cm in groundwater suggesting fresh nature in terms of its salinity. In the study, As and Pb could not be detected by ICS-AES, Cu and Zn were below the Bureau of Indian Standards acceptable limit for drinking water (IS 10500, 1991) and Fe and Mn exceed 28 and 136 times more, respectively, than the US Environmental Protection Agency (EPA 2006) limit for drinking water. The distribution of concentrations of these ions in surface water and groundwater were found in the order of Mn > Fe > Zn > Cu > Pb > As. In general, the ion concentrations of Fe and Mn in deep groundwater show the highest concentrations (19.537 mg/l and 19.170 mg/l, respectively) followed by shallow groundwater (5.324 mg/l and 6.308 mg/l, respectively). Fe and Mn ions in river water have the lowest concentrations (9.059 mg/l and 0.764 mg/l, respectively) while, ponds and lakes have the moderately high concentrations (16.222 mg/l and 7.246 mg/l for ponds and 13.305 mg/l and 4.242 mg/l for lake, respectively).
Correlation matrices
Correlation analysis is a bivariate method which simply exhibits how well one variable predicts the other and has been widely using in interpreting hydrochemical data (Usunoff and Guzman–Guzman 1989; Adams et al. 2001; Tariq et al. 2006; Chen et al. 2007; Kshetrimayum 2015). The results of correlation matrices for Fe, Mn, TDS, Zn, Cu, pH variables for surface water and groundwater samples are shown in Table 2. Samples showing r > 0.7 are considered to be strongly correlated whereas r > 0.5–0.7 are considered moderately correlated at a significance level (p) of <0.01 (2-tailed) and <0.05 (2-tailed). There is strong positive correlation between Fe and Mn (r = 0.925 and r = 0.665 for surface water and groundwater, respectively) indicating similar sources of reduction under anoxic and redox oscillating conditions (Postma 1985; Hem 1989; Nealson and Myers 1992; Postma and Appelo 2000; Buschmann et al. 2007). Moderate positive correlation exists among TDS with Fe and Mn (r = 0.557 and 0.474, respectively, in surface water and r = 0.271 and 0.493, respectively, in groundwater). These relations may indicate that the elevated ionic strength and inorganic complexes can drive more Fe and Mn ions into solution (Stumm and Morgan 1981). Evaporation from lake, surface pond and shallow groundwater could be important in elevating the concentrations of dissolved constituents in the water. Combination of the high TDS and the reductive hydrochemistry causes distribution of the high concentration zone of Fe and Mn (Weng et al. 2007). There is a weak negative correlation among Cu with EC, Fe, Mn (r = −0.379, r = −0.216, r = −0.252, respectively in surface water and r = −0.003, r = −0.267, r = −0.252, respectively, in groundwater). Another strong negative correlation exists between Zn and EC (r = −0.762) in surface water. The negative correlation, though weak, exists among Zn with Fe, Mn (r = −0.101, r = −0.135, respectively) in surface water. Weak negative correlation has also observed among Zn with EC, Fe, Mn (r = −0.15, r = −0.247, r = −0.029, respectively). These relations suggest that dissolution of silicate minerals and sorption by stream sediments in groundwater (Hem 1989) in oxidized condition curtailed the reduction of Fe2+ and Mn2+ under redox environment and vice versa. A weak positive correlation exists between Zn and Cu (r = 0.406 and r = 0.012 in surface water and groundwater, respectively) indicating similar sources of geogenic origin.
Factor analysis
Factor analysis is a tool used to rearrange data to present it in a manner that better explains the structure of the underlying system that produced the data (Dawdy and Feth 1967) and has been widely used in analyzing interrelations among hydrochemical parameters (Ashley and Lloyd 1978; Usunoff and Guzman-Guzman 1989; Schot and Van der Wal 1992; Adams et al. 2001; Farnham et al. 2003; Chen et al. 2007). The hydrochemical data were analyzed using Principal Component Analysis (PCA) to study the possible processes responsible for the groundwater chemistry in the study area. Three factors, with eigen values >1 with a cumulative variance of more than 79 % have been identified (Table 3). PC1 exhibits high loading for TDS, EC, pH and Mn with a maximum contribution of 33.87 % of total variance. PC2 shows high loading for Fe, Cu, and Mn with a contribution of 23.74 % of total variance. PC3 exhibits high loading for pH and Zn with a contribution of 21.90 % of total variance. The two greatest positive PC1 loadings (EC-0.907; TDS-0.959) occurred as soluble salts in oxidizing water whereas one negative loading (pH-0.64) occurred within oxygen depleted groundwater. The greatest positive PC2 loadings (Fe-0.807; Mn-0.646) exhibited more soluble in oxidizing alkaline environment water (Hem 1989; Farnham et al. 2003). The highest negative PC3 loading (Zn-0.870) occurred as soluble oxyanions in oxidizing water. PC1 and PC3 may indicate oxidizing condition which may control occurrence of salt minerals and some trace metals. PC2 reflects reducing environment in groundwater and controlled the elevated Fe and Mn concentrations. Component plot in rotated space (Fig. 2) of PC1, 2 and 3 also indicate that trace metals with greatest positive PC loadings were abundant in more reducing environment.
Groundwater flow directions and Fe and Mn distribution
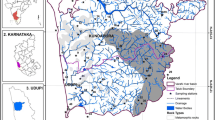
The detail information of 36 openwells which were used to identify groundwater flow direction and areas of recharge and discharge is given in Table 4. As depicted by the water table elevation contour map (Fig. 3), the depth to water table and water table elevation varies from 1.52 m bgl to 10.75 m bgl and 123.64 m amsl (above mean sea level) to 179.27 m amsl, respectively. The regional groundwater flow direction has been identified as south to north. The flow net pattern suggests that the Chathe river acts as influent stream which discharge into the surrounding water table. Based on flow directions, two groundwater recharge zones have been identified: (a) around upstream of the Chathe river and (b) the Chathe and Dhansiri river confluence zone. Groundwater in these recharge zones has high Fe and Mn concentrations. Baseflow from the discharge areas into low lying areas (~132 m) causes accretion of Fe and Mn in pond, oxbow lake, backswamp and shallow aquifers. Moderately high Fe concentrations (10 mg/l–20 mg/l) were identified in the Dhansiri flood plain which is occupied by Recent alluvium deposit of silt, clay and sand, piedmont areas which are mainly composed of ferruginous sandstone, conglomerate and shale of Surma Group of Lower Miocene age and backswamp area (Figs. 4, 5). The exceedingly high Fe concentrations (>20 mg/l) were observed at upstream and downstream of the Chathe river, the Dhansiri and Chathe flood plains and backswamp area. In case of Mn, moderately high concentrations (0.1 mg/l–5 mg/l) were observed around the Dhansiri and Chathe floodplains, upstream and downstream of the Chathe river. The excessively elevated levels (5 mg/l to >20 mg/l) of Mn were observed at upper piedmont, floodplains of the Dhansiri and Chathe rivers and backswamp area. In deep wells and springs, where both the oxygen content and pH tend to be low, water containing dissolved Fe and Mn appears colorless. When exposed to air, the dissolved Fe or Mn reacts with oxygen and is converted to a colored, solid material that settles out of the water. Fe forms reddish brown while Mn forms a black or deep brown residue. These coloured stains were commonly observed around the shallow hand pumped wells and along river cuttings in the study area.
Discussion
To study the possible sources of Fe and Mn elevations in the study area, results of correlations, spatial distribution of Fe and Mn, local and regional groundwater flow directions were considered to arrive at an empirical result. The cross plot of Fe and pH (Fig. 6a) indicates that most of the Fe elevated water samples have pH less than 7. Among the high elevated Fe samples, those collected from deep and shallow groundwater and surface water bodies like backswamp, oxbow lake, pond have acidic water and only those collected from few of shallow groundwater have alkaline water. The similar pattern has been observed for Mn in the cross plot (Fig. 6b). The relationship between Fe and Mn on cross plot (Fig. 6c) shows that most of the samples curtailed their concentrations within 35 mg/l and 15 mg/l for Fe and Mn, respectively. These relationships implies that (1) the redox condition and pH are critical factors that dominate the migration of Fe and Mn into groundwater (Hem 1989; Brown et al. 1999) (2) the solubility of Mn as Mn2+ must have been high in low pH waters and much lower in oxidizing water because Mn precipitates as Mn4+ oxides from solution in more oxidizing waters (Hem 1989; Farnham et al. 2003) (3) Mn which is rarely found in a water source alone is found in conjunction with dissolved Fe and shows a strong correlation. These observations were reinforced by the presence of high Fe and Mn concentrations in the deep groundwater and less in shallow groundwater samples (Table 1). The microbial metabolism of organic carbon in sediments drives reduction of both Fe hydroxides and Mn oxides (Myers and Nealson 1988; Lovley 1997; Nealson 1997; Lovely and Phillips Lovley and Phillips 1988; Chapelle 2000). The possible sources of dissolved organic carbon (DOC) include natural solid organic matter in aquifers (Ravenscroft et al. 2001; Mladenov et al. 2010), waste organic matter in in-situ sanitation (McArthur et al. 2004), and degrading hydrocarbons (Rowland et al. 2006). The present study area is located around Upper Assam basin where several hydrocarbon deposits are located (Saikia and Dutta 1980; Handique and Bharali 1981; Krishna Rao and Parasad 1982; Raju and Mathur 1995; Kent et al. 2002) and DOC from these deposits may act as redox driver for both Fe and Mn in groundwater. The runoff and baseflow from the recharge areas into low lying areas causes accretion of Fe and Mn in pond, oxbow lake, backswamp and shallow aquifers. Our study also observed that high elevation of Fe and Mn accumulations in surface water body like backswamp, pond, oxbow lake and shallow groundwater has been controlled by groundwater flow directions. Fe and Mn contaminated water from the Chathe river discharges as influent river into shallow aquifers around the flood plains. The upstream of Dhansiri and Chathe rivers flows through rocks like Tipam sandstone, Girujan clay of Tipam Group and shales, siltsones, clays of Surma Group. These rocks formed a thrust known as Naga Thrust where several deposits of hydrocarbons are known to be present. The presence of organic matters in these rock types under suitable geogenic conditions enhances dissolution of Fe and Mn in the groundwater.
Conclusions
The present state of Fe and Mn elevations in surface water and groundwater around tectonically active Naga foothills located in a transitional crust was found to be highly alarming. 32 water samples were collected for the study and found all of them well above the EPA limit. Fe and Mn concentrations exceed 28 and 136 times more, respectively, than this limit. The moderate positive correlation among TDS with Fe and Mn indicated elevated inorganic complexes leading to high Fe and Mn ions in solution. Negative correlation among Cu and Zn with Fe and Mn suggested that dissolution of silicate minerals and sorption by stream sediments in groundwater in oxidized condition curtail the reduction of Fe2+ and Mn2+ under redox environment and vice versa. A positive correlation between Zn and Cu indicated similar source of geogenic origin. The majority of the Fe and Mn elevated water samples showed low pH with a strong correlation between Fe and Mn suggesting reduction of both Fe hydroxides and Mn oxides into dissolve states under similar environment. Out of 3 PC factors, PC1 and PC3 reflected oxidized condition in groundwater and PC2 reflected reducing environment that controlled elevations of Fe and Mn concentrations in groundwater. The study also revealed that source of high Fe and Mn can be controlled by groundwater flows. The elevated Fe and Mn water from the Dhansiri and the Chathe rivers discharge the surrounding water table. Groundwater with elevated Fe and Mn in recharge zones supply water to the low lying discharge areas like backswamp, oxbow lake, pond and shallow groundwater leading to high elevation of Fe and Mn concentrations. Thus, from the present study it has been concluded that the sources for high elevated Fe and Mn concentrations in shallow groundwater and surface water bodies in the study area may be identified as discharge of the Dhansiri and Chathe river water into the surrounding water table, baseflow discharge into the low lying areas and dissociation of Fe and Mn oxides by organic metabolism under reduced environment.
References
Adams S, Titus R, Pietersen K, Tredoux G, Harris C (2001) Hydrochemical characteristics of aquifers near Sutherland in the Western Karoo, South Africa. J Hydrol 241:91–103
Alam S, Kodama R, Akiha F, Kamei S, Kawai S (2006) All eviation of manganese phytotoxicity in barley with calcium. J Plant Nutrit 29:59–74
APHA (2005) Standard methods for the examination of water and waste water, American Health Public Association, WWA, 21st edn. Washington, DC
Ashley RP, Lloyd JW (1978) An example of the use of factor analysis and cluster analysis in groundwater chemistry interpretation. J Hydrol 39:355–364
Bhandari LL, Fuloria R, Sastry VV (1973) Stratigraphy of Assam valley, India. Am Assoc Petrol Geol Bull 57:642–652
Borah KK, Bhuyan B, Sarma HP (2009) Lead, Arsenic, fluoride, and iron contamination of drinking water in the tea garden belt of Darrang district, Assam, India. Environ Monit Assess 169:347–352
Bouchard M, Laforest F, Vandelac L, Bellinger D, Mergler D (2007) Hair manganese and hyperactive behaviors: pilot study of school age children exposed through tap water. Environ Health Perspect 115:122–127
Bouchard MF, Sauve S, Barbeau B, Legrand M, Brodeur ME, Bouffard T, Limoges E, Bellinger DC, Mergler D (2011) Intellectual impairment in school-age children exposed to manganese from drinking water. Environ Health Perspect 119:138–143
Brown CJ, Walter DA, Colabufo S (1999) Fe in the aquifer system of Suffolk County, New York. US Geol Surv Wat Resour Invest Rep. 10:99–4126
Buragohain M, Bhuyan B, Sarma HP (2010) Seasonal variations of lead, arsenic, cadmium and aluminium contamination of groundwater in Dhemaji district, Assam, India. Envion Monit Assess 170:345–351
Buschmann J, Berg M, Stengel C, Sampson M (2007) Arsenic and manganese contamination of drinking water resources in Cambodia: coincidence of risk areas with low relief topography. Environ Sci Technol 41:2146–2156
Chakrabarty S, Sarma HP (2011) Heavy metal contamination of drinking water in Kamrup district, Assam, India. Environ Monit Assess 179:479–486
Chapelle FH (2000) The significance of microbial processes in hydrogeology and geochemistry. Hydrogeol J 8:41–46
Chen K, Jiao JJ, Huang J, Huang R (2007) Multivariat statistical evaluation of trace elements in groundwater in a coastal area in Shenzen, China. Environ Pollu 147:771–780
Corps EV (1949) Digboi oil field, Assam. AAPG Bull 33:1–21
Davis SN, Dewiest RJM (1966) Hydrogeology. Wiley, New york p 463
Dawdy DR, Feth JH (1967) Application of factor analysis in study of chemistry of groundwater quality, Mojaveriver Valley California. Water Resour Res 3:505–510
Edmunds WM, Kinniburgh DG, Moss PD (1992) Trace metals in interstitial waters from sandstones: acidic inputs to shallow groundwaters. Environ Pollut 77:129–141
Environmental Protection Agency, USA (EPA) (2006) Ground water and drinking water: current drinking water standards. http://www.epa.gov/safewater/mcl.html. Accessed 24 Feb 2015
Farnham IM, Johannesson KH, Sing AK, Hodge VF, Stetzenbach KJ (2003) Factor analytical approaches for evaluating groundwater trace elements chemistry data. Anal Chim Acta 490:123–138
Gilkes RJ, McKenzie RM (1988) Geochemistry of manganese in soils. In: Graham RD, Hannam RJ, Uren NC (eds) Manganese in soils and plants. Kluwer, Dordrecht, pp 23–35
Hafeman D, Litvak P, Cheng Z, van Geen A, Ahsan H (2007) Association between manganese exposure through drinking water and infant mortality in Bangladesh. Environ Health Perspect 115:1107–1112
Haloi N, Sarma HP (2012) Heavy metal contaminations in the groundwater of Brahmaputra flood plain: an assessment of water quality in Barpeta District, Assam (India). Environ Monit Assess 184:6229–6237. doi:10.1007/s10661-011-2415-x
Handique GK, Bharali B (1981) Temperature distribution and its relation to hydrocarbon accumulation in upper Assam Valley, India. AAPG Bulletin 65:1633–1641
Hem JD (1989) Study and interpretation of the chemical characteristics of natural water, USGS Water Supply Paper 2254,263
Horsley SB, Long RP, Bailey SW, Hallett RA, Hall TJ (2000) Factors associated with the decline disease of sugar maple on the Allegheny Plateau. Can J For Res 30:1365–1378
Jaiswal (2009) Seismic imaging of the Naga Thrust using multiscale waveform inversion. Geophysics 74:129–140
Jenne EA (1968) Controls on Mn, Fe Co, Ni, Cu and Zn concentrations in soils and waters: the significant role of hydrous Mn and Fe oxides. Am Chem Soc 337–387
Kent WN, Hickman RG, Dasgupta U (2002) Application of a ramp/flat fault model to interpretation of the Naga thrust and possible implications for petroleum exploration along the Naga thrust front. AAPG Bull 86:2023–2045
Khan S, Qureshi MA, Singh JB (1996) Studies on the mobility of heavy metals in soil. Indian J Environ Health 38:1–6
Kogelmann WJ, Sharpe ES (2006) Soil acidity and manganese in declining and nondeclining sugar maple stands in Pennsylvania. J Environ Qual 35:433–441
Kondakis XG, Makris N, Leotsinidis M, Prino M, Papapetropoulos T (1989) Possible health effects of high manganese concentration in drinking water. Arch Environ Health 44:175–178
Kraft GS, Stites W, Mechenich DJ (1999) Impacts of irrigated vegetable agriculture on a humid north–Central US, Sand plain aquifer. Groundwater 37:572–580
Krishna Rao VV, Parasad KL (1982) Exploration in the “schuppen belt” of Nagaland. Bull Oil Natural Gas Comm 19:213–220
Kshetrimayum KS (2015) Hydrochemical evaluation of shallow groundwater aquifers: a case study from a semiarid Himalayan foothill river basin, northwest India. Environ Earth Sci 74:7187–7200
Liap Z (1992) Environmental chemistry of trace elements and their biological effect. Chinese Environmental Science Press, Beijing, p 320
Ljung K, Vahter M (2007) Time to re-evaluate the guideline value for manganese in drinking water? Environ. Health Perspect 15:1533–1538
Lovley DR (1997) Microbial Fe(III) reduction in subsurface environments. Microbiol Rev 30:305–313
Lovley DR, Phillips EJP (1988) Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol 54:1472–1480
Manceau A, Gorshkov AI, Drits VA (1992) Structural chemistry of Mn, Fe Co, and Ni in manganese hydrous oxides: part II. Information from EXAFS spectroscopy and electron and X-ray diffraction. Am Mineral 77:1144–1157
Mathur LP and Evans P (1964) Oil in India: 22nd Session International Geological Congress Proceedings, pp. 7–52
McArthur JM, Banerjee DM, Hudson-Edwards KA, Mishra R, Purohit R, Ravenscroft P, Cronin A, Howarth RJ, Chatterjee A, Talukder T, Lowry D, Houghton S, Chadha DK (2004) Natural organic matter in sedimentary basins and its relation arsenic in anoxic ground water: the example of West Bengal and its worldwide implications. Appl Geochem 19:1255–1293
McArthur JM, Sikdar PK, Nath B, Grassineau N, Marshall JD, Banerjee DM (2012) Sedimentological control on Mn, and other trace elements, in groundwater of the Bengal delta. Environ Sci Technol 46:669–676
Mladenov N, Zheng Y, Miller MP, Nemergut D, Legg T, Simone B, Hageman C, Moshiurrahaman M, Ahmed KM, McNight D (2010) Dissolved organic matter sources and consequences for iron and arsenic mobilization in Bangladesh aquifers. Environ Sci Technol 44:123–128
Myers CR, Nealson KH (1988) Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science 240:1319–1321
Nealson KH (1997) Sediment bacteria: who’s there, what are they doing, and what’s new? An Rev Earth Planet Sci 25:403–434
Nealson KH, Myers CR (1992) Microbial reduction of manganese and iron: new approaches to carbon cycling. Appl Environ Microbiol 58:439–443
Nriagu JO, Pacyna JM (1988) Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature 333:134–139
Pawar NJ, Nikumbh JD (1999) Trace element geochemistry of groundwater from Behedi Basin, Nasik district, Maharashtra. J Geol Soc India 54:501–514
Post JE (1999) Manganese oxide minerals: crystal structures and economic and environmental significance. Proc Natl Acad Sci 96:3447–3454
Postma D (1985) Concentration of Mn and separation from Fe in sediments—I. Kinetics and stoichiometry of the reaction between birnessite and dissolved Fe(II) at 10 °C. Geochim Cosmochim Acta 49:1023–1033
Postma D, Appelo CAJ (2000) Reduction of Mn-oxides by ferrous iron in a flow system: column experiment and reactive transport modeling. Geochim Cosmochim Acta 64:1237–1247
Raju SV, Mathur N (1995) Petroleum geochemistry of a part of upper Assam basin, India: a brief overview. Org Geoch 23:55–70
Ravenscroft P, McArthur JM, Hoque B (2001) Geochemical and palaeohydrological controls on pollution of groundwater by arsenic. In: Chappell WR, Abernathy CO, Calderon R (eds) Arsenic exposure and health effects IV. Elsevier Science Ltd, Oxford, pp 53–77
Romic M, Romic D (2003) Heavy metals distribution in agricultural topsoils in urban area. Environ Geol 43:795–805
Rowland HAL, Polya DA, Lloyd JR (2006) Characterisation of organic matter in a shallow, reducing, arsenic-rich aquifer, West Bengal. Org Geochem 37:1101–1114
Saikia MM, Dutta TK (1980) Depositional environment of source beds of high-wax oils in Assam basin, India. AAPG Bull 64:427–430
Sanders CJ, Santos IR, Barcellos R, Filho EVS (2012) Elevated concentrations of dissolve Ba, Fe and Mn in a mangrove subterranean estuary: consequence of seal level rise? Cont Shelf Res 43:86–94
Schot PP, Van der Wal J (1992) Human impact on regional groundwater composition through intervention in natural flow patterns and changes in land use. J Hydrol 134:297–313
Spangler AH, Spangler JG (2009) Groundwater manganese and infant mortality rate by county in North Carolina: an ecological analysis. EcoHealth 6:596–600
Sparrow LA, Uren NC (1987) The role of manganese toxicity in crop yellowing on seasonally waterlogged and strongly acidic soils in northeastern Victoria. Aust J Exp Agric 27:303–307
Stumm W, Morgan JJ (1981) Aquatic chemistry: an introduction emphasizing chemical equilibria in natural waters. Wiley, New York, p 554
Suarez DL, Langmuir D (1976) Heavy metal relationships in a Pennsylvania soil. Geochim Cosmochim Acta 40:589–598
Subba Rao N (1993) Environmental impact of industrial effluents in groundwater regime of Visakhapatnam industrial complex. Indian J Geol 65:35
Subba Rao N, Madhusudhana Reddy P (2006) Monitoring the groundwater quality in an urban area: an environmental impact assessment and management. J Appl Geochem 8:37–56
Tariq SR, Shah MH, Shaheen N, Khalique A, Manzoor S, Jaffar M (2006) Multivariate analysis of trace metal levels in tannery effluents in relation to soil and water: a case study from Peshawar, Pakistan. J Environ Manag 79:20–29
Usunoff EJ, Guzman-Guzman A (1989) Multivariate analysis in hydrochemistry: an example of the use of factor and correspondence analysis. Ground Water 27:27–34
Wasserman GA, Liu X, Parvez F, Ahsan H, Levy D, Litvak PF, Kline J, van Geen A, Slavkovich V, LoIacono NJ, Cheng Z, Zheng Y, Graziano JH (2006) Water manganese exposure and children’s intellectual function in Araihazar, Bangladesh. Environ Health Perspect 114:12–129
Wasserman GA, Liu X, Parvez F, Litvak PF, Ahsand H, Levy D, Kline J, van Geen A, Mey J, Slavkovich V, Siddique AB, Islam T, Graziano JH (2011) Arsenic and manganese exposure and children’s intellectual function. Neurotoxicology 32:450–457
Weng HX, Qin YC, Chen XH (2007) Elevated iron and manganese concentrations in groundwater derived from the Holocene transgression in the Hang-Jia-Hu Plain, China. Hydrogeol J 15:715–726
WHO (2005) Nutrients in drinking water, World Health Organisation Library Cataloguing-in-Publication Data. ISBN 9241593989
Acknowledgments
The authors would like to thank Prof N. Pandey, Department of Earth Science, Assam University for sharing his knowledge on geology of NE India. The authors are thankful to the anonymous reviewers for suggestions to improve the understanding of the present manuscript. One of the authors Miss Heizule Hegeu acknowledges Union Grant Commission for providing junior research fellowship Grant to conduct the present study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kshetrimayum, K.S., Hegeu, H. The state of toxicity and cause of elevated Iron and Manganese concentrations in surface water and groundwater around Naga Thrust of Assam-Arakan basin, Northeastern India. Environ Earth Sci 75, 604 (2016). https://doi.org/10.1007/s12665-016-5372-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-016-5372-4