Abstract
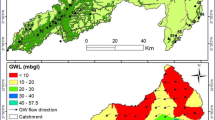
The present study aimed at identifying the salinity source in the groundwater of Lenjanat Plain. To do so, non-isotopic geochemical methods were employed: groundwater samples were collected seasonally from 33 wells widespread in the area, and physicochemical parameters as well as major and minor elements were measured in the 132 samples. The data collected from the field and laboratory measurements were interpreted through statistical and hydrogeochemical graphs, mass ratios and saturation indexes obtained from modeling. The results revealed that hydrogeochemical properties of the study aquifer were controlled by rock/water interactions including ion exchange, dissolution of evaporation deposits (halite and gypsum) and precipitation/dissolution of carbonates. Based on the values of Cl/Br ratio in Lenjanat groundwater (329–4,492), dissolution of evaporation deposits in aquifer was the main cause for groundwater salinity. Considering the Lenjanat groundwater geochemical properties, the data confirm the reported Cl/Br ratios for groundwater affected by the dissolution of evaporation deposits (Cl/Br > 300) and overlaps with the range of Cl/Br ratios for domestic sewage effluent groundwater. Selecting the best chemical components and their ratios in non-isotopic geochemical methods provides an accurate distinction between sources of groundwater salinity.















Similar content being viewed by others
References
Alley WM (1993) Regional groundwater quality. Van Nostrand Reinhod, New York
APHA (2005) Standard methods for the examination of water and wastewater. American Public Health Association (APHA), 21st edn. American Water Works Association (AWWA) and Water Environment Federation (WEF), Denver, New Jersey
Cartwright I, Weaver TR, Fifield LK (2006) Cl/Br ratios and environmental isotopes as indicators of recharge variability and groundwater flow. An example from the southeast Murray Basin, Australia. Chem Geol 231:38–56
Cartwright I, Hall Sh, Tweed S, Leblanc M (2009) Geochemical and isotopic constraints on the interaction between saline lakes and groundwater in southeast Australia. Hydrogeol J 17:1991–2004
Davis SN, Whittemore DO, Fabryka-Martin J (1998) Uses of chloride/bromide ratios in studies of potable water. Groundw J 36:338–350
Davis SN, Cecil LD, Zreda M, Moysey S (2001) Chlorine-36, bromide, and the origin of spring water. Chem Geol 179:3–16
Edmunds WM, Shand P (2008) Natural groundwater quality. Blackwell, Oxford
Edmunds WM, Smedley PL (2000) Residence time indicators in groundwater: the East Midlands Triassic sandstone aquifer. Appl Geochem 15:737–752
Fetter CW (2000) Applied hydrogeology. CBS Publishers, New Delhi
Fisher RS, Mullican WF (1997) Hydrochemical evolution of sodium-sulfate and sodium-chloride groundwater beneath the northern Chihuahuan Desert, Trans-Pecos, Texas, USA. Hydrogeol J 5:4–16
Geological Survey of Iran (1976a) Geological Map of Iran 1:100000 Series, Sheet 6354 Shahreza. Ministry of Industry and Mines
Geological Survey of Iran (1976b) Geological Map of Iran, 1:100000 Series, Sheet 6254 Riz-e-Lenjan. Ministry of Industry and Mines
Gibbs RJ (1970) Mechanism controlling world water chemistry. Sci 170:795–840
GS US (2006) Field measurement TWRI Book 9. United State Geological Survey, Reston
Hopkins DG, Richardson JL (1999) Detecting a salinity plume in an unconfined sandy aquifer and assessing secondary soil salinization using electromagnetic induction techniques, North Dakota USA. Hydrogeol J 7:380–392
Hounslow AW (1995) Water quality data, analysis and interpretation. Lewis Publication, Boca Raton
Hudak PF (2003) Chloride/bromide ratios in leachate derived from farm-animal waste. Environ Pollut 121:23–25
Iran Steel Co (1973) Geological and lithological section of wells. Department of Engineering Geology
Jafarian MA (1985) The project of soils studies in Mobarakeh Steel Co. Isfahan University, Isfahan
Langmuir D (1997) Aqueous environmental geochemistry. Prentice-Hall, New Jersey
Mariel A, Vengosh A (2001) Sources of salinity in ground water from Jericho area, Jordan valley. Groundw J 39:240–248
Ministry of Energy (1985) The report of geophysical studies in Mobarakeh (Isfahan). Department of Groundwater
Paine JG (2003) Determining salinization extent, identifying salinity sources, and estimating chloride mass using surface, borehole, and airborne electromagnetic induction methods. Water Resour Res 39:1–10
Parkhurst DL, Appelo CAJ (2005) PHREEQC (Version 2.18). A Hydrogeochemical Transport Model
Parsabe Sepahan Andish (2008) The project of contaminants recognition in Zarinshahr. Thirteen Volumes Zarinshahr Municipality
Richter BC, Kreitler CW (1992) Identification of sources of ground-water salinization using geochemical techniques. U.S. Environmental Protection Agency, Washington, DC
Richter BC, Kreitler CW (1993) Geochemical techniques for identifying source of ground-water salinization. C.K.Smoley, Chelsea
Slater LD, Binley A, Brown D (1997) Electrical imaging of fractures using ground-water salinity change. Groundw J 35:436–442
Stuyfzand PJ (1989) A new hydrochemical classification of water types. IAHS Publ No 182
USEPA (2007) Groundwater sampling SESDPROC-301-R1, SESD operating procedure for groundwater sampling. United State Environmental Protection Agency, Washington, DC
Vengosh A, Pankratov I (1998) Chloride/bromide and chloride/fluoride ratios of domestic sewage effluents and associated contaminated ground water. Groundw J 36:815–824
Younger PL (2007) Groundwater in the environment: an introduction. Blackwell, Oxford
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nassery, H.R., Kayhomayoon, Z. Source of salinity in the groundwater of Lenjanat Plain, Isfahan, Iran. Environ Earth Sci 68, 413–427 (2013). https://doi.org/10.1007/s12665-012-1746-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-012-1746-4