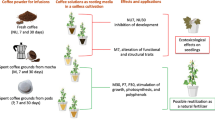
Abstract
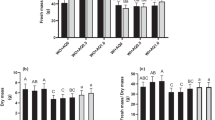
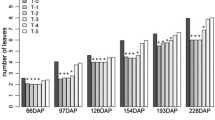
Spent coffee grounds (SCG) and spent tea leaves (STL) are constantly rejected either on a domestic scale or more widely on an industrial level. The main objective of this work was to evaluate the potential use of their aqueous extracts as organic priming agents in pepper (Capsicum annuum L.) in vitro germination under salt (NaCl, 102.66 mM) or drought (PEG 6000, 4%) stress for 384 h. Extraction from SCG and STL were achieved by three conventional techniques (infusion, boiling and incubation at room temperature). Thereafter, different concentrations from each by-product were examined. The results showed that, under salt stress, STL extract at 1.5% dry tea leaves (DTL), obtained by infusion extraction, displayed the best response in terms of final germination percentage (FGP), time taken for cumulative germination to reach 50% of its maximum (t50) and germination uniformity (U7525), whereas, the best improve in root length was obtained with 2.25% DTL compared with unprimed culture. Against drought stress, SCG extract at 4% dry coffee ground (DCG), obtained following incubation extraction, resulted in amelioration in FGP and U7525, however, 2% DCG engendered the best stimulation of growth. Added directly to the medium culture, STL (1.5% DTL) caused a highly significant inhibition of the germination process with both types of stress. However, SCG (2% DCG) supplementation is recommended mainly in drought stress to improve seedlings growth. Organic priming and medium supplementation can be alternative pathways for SCG and STL wastes valorization. Furthermore, these techniques can be a good cropping practice to improve seed potentiality against environmental challenges in sustainable agriculture.
Graphic Abstract









Similar content being viewed by others
References
Ramalakshimi, K., Rao, L.J.M., Takano-Ishikawa, Y., Goto, M.: Bioactivities of low-grade green coffee and spent coffee in different in vitro model systems. Food Chem. 115, 79–85 (2009)
Murthy, P.S., Naidu, M.M.: Recovery of phenolic antioxidants and functional compounds from coffee industry by-products. Food Bioprocess. Technol. 5, 897–903 (2010)
Zuorro, A., Lavecchia, R.: Spent ground coffee as valuable source of phenolic compounds and bioenergy. J. Clean. Prod. 34, 49–56 (2012)
Faraha, A., Donangelo, C.M.: Phenolic compounds in coffee. Braz. J. Plant Physiol. 18, 23–36 (2006)
Dai, J., Mumper, R.J.: Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules 15, 7313–7352 (2010)
Esquivel, P., Jiménez, V.M.: Functional properties of coffee and coffee by-products. Food Res. Int. 46, 488–495 (2011)
Ronga, D., Pane, C., Zaccardelli, M., Pecchioni, N.: Use of spent coffee ground compost in peat-based growing media for the production of basil and tomato potting plants. Commun. Soil Sci. Plant Anal. 47, 356–368 (2016)
Yamane, K., Kono, M., Fukunaga, T., Iwai, K., Sekine, R., Watanabe, Y., Iijima, M.: Field evaluation of coffee grounds application for crop growth enhancement, weed control, and soil improvement. Plant Prod. Sci. 17, 93–102 (2014)
Ciesielczuk, T., Rosik-Dulewska, C., Poluszyńska, J., Miłek, D., Szewczyk, A., Sławińska, I.: Acute toxicity of experimental fertilizers made of spent coffee grounds. Waste Biomass Valoriz. 9, 2157–2164 (2017)
Hardgrove, S.J., Livesley, S.J.: Applying spent coffee grounds directly to urban agriculture soils greatly reduces plant growth. Urban For. Urban Green. 18, 1–8 (2016)
Sant’Anna, V., Biondo, E., Kolchinski, E.M., da Silva, L.F.S., Corrêa, A.P.F., Bach, E., Brandelli, A.: Total polyphenols, antioxidant, antimicrobial and allelopathic activities of spend coffee ground aqueous extract. Waste Biomass Valoriz. 8, 439–442 (2017)
Pan, X., Niu, G., Liu, H.: Microwave-assisted extraction of tea polyphenols and tea caffeine from green tea leaves. Chem. Eng. Process. 42, 129–133 (2003)
Hameed, B.H.: Spent tea leaves: a new non-conventional and low-cost adsorbent for removal of basic dye from aqueous solutions. J. Hazard. Mater. 161, 753–759 (2009)
Zuorro, A., Lavecchia, R., Medici, F., Piga, L.: Spent tea leaves as a potential low-cost adsorbent for the removal of azo dyes from wastewater. Chem. Eng. Trans. 32, 19–24 (2013)
Akar, E., Altinisik, A., Seki, Y.: Using of activated carbon produced from spent tea leaves for the removal of malachite green from aqueous solution. Chem. Eng. J. 52, 19–27 (2013)
Farhoosh, R., Golmovahhed, G.A., Khodaparast, M.H.H.: Antioxidant activity of various extracts of old tea leaves and black tea wastes (Camellia sinensis L.). Food Chem. 100, 231–236 (2007)
Tanou, G., Fotopoulos, V., Molassiotis, A.: Priming against environmental challenges and proteomics in plants: update and agricultural perspectives. Front. Plant Sci. 3, 216 (2012)
Paparella, S., Araújo, S.S., Rossi, G., Wijayasinghe, M., Carbonera, D., Balestrazzi, A.: Seed priming: state of the art and new perspectives. Plant Cell Rep. 34, 1281–1293 (2015)
Hamed, M., Kalita, D., Bartolo, M.E., Jayanty, S.S.: Capsaicinoids, polyphenols and antioxidant activities of Capsicum annuum: comparative study of the effect of ripening stage and cooking methods. Antioxidants 8, 364 (2019)
Gammoudi, N., San Pedro, T., Ferchichi, A., Gisbert, C.: Improvement of regeneration in pepper: a recalcitrant species. In Vitro Cell Dev. Biol. Plant 54, 145–153 (2018)
Khan, H.A., Pervez, M.A., Ayub, C.M., Ziaf, K., Bilal, R.M., Shahid, M.A., Akhtar, N.: Hormonal priming alleviates salt stress in hot pepper (Capsicum annuum L.). Soil Environ. 28, 130–135 (2009)
Korkmaz, A., Şirikçi, R.: Improving salinity tolerance of germinating seeds by exogenous application of glycinebetaine in pepper. Seed Sci. Technol. 39, 377–388 (2011)
Aloui, H., Souguir, M., Latique, S., Hannachi, C.: Germination and growth in control and primed seeds of pepper as affected by salt stress. Cercet. Agron. Mold. 47, 83–95 (2014)
Gammoudi, N., Karmous, I., Zerria, K., Loumerem, M., Ferchichi, A., Nagaz, K.: Efficiency of pepper seed invigoration through hydrogen peroxide priming to improve in vitro salt and drought stress tolerance. Hortic. Environ. Biotechnol. (2020). https://doi.org/10.1007/s13580-020-00260-8
Mavi, K., Atak, M., Atış, I.: Effect of organic priming on seedling emergence of pepper under salt stress. Soil-Water J 2, 401–408 (2013)
Mavi, K.: The effect of organic priming with marigold herbal tea on seeds quality in aji pepper (Capsicum baccatumvar. pendulum Willd.). MKU J. Agric. Fac. 21, 31–39 (2016)
Mavi, K.: Evaluation of organic priming to improve the emergence performance of domesticated Capsicum species. Seed Sci. Technol. 46, 131–137 (2018)
Dutta, S.K., Layek, J., Akoijam, R.S., Boopathi, T., Saha, S., Singh, S.B., Prakash, N.: Seaweed extract as natural priming agent for augmenting seed quality traits and yield in Capsicum frutescens L. J. Appl. Phycol. 31, 3803–3813 (2019)
Gammoudi, N., Zerria, K., Nagaz, K., Ferchichi, A.: Enhancement of capsaicinoids in vitro production by abiotic elicitors in placenta-derived callus of Capsicum annuum L. Tunisian var. ‘Baklouti Medenine’. Biologia 74, 725–732 (2019)
Murashige, T., Skoog, F.: A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 15, 473–497 (1962)
Hachicha, M., Ben Aissa, I.: Managing salinity in Tunisian oases. Life Sci. J. 8, 775–782 (2014)
El-Kassaby, Y.A., Moss, I., Kolotelo, D., Stoehr, M.: Seed germination: mathematical representation and parameters extraction. For. Sci. 54, 220–227 (2008)
Joosen, R.V., Kodde, J., Willems, L.A., Ligterink, W., van der Plas, L.H., Hilhorst, H.W.: GERMINATOR: a software package for high-throughput scoring and curve fitting of Arabidopsis seed germination. Plant J. 62, 148–159 (2010)
Abdul-baki, A.A., Anderson, J.D.: Vigor determination in soybean by multiple criteria. Crop Sci. 13, 630–633 (1973)
Rosental, L., Nonogaki, H., Fait, A.: Activation and regulation of primary metabolism during seed germination. Seed Sci. Res. 24, 1–15 (2014)
Huang, W.Y., Lin, Y.R., Ho, R.F., Liu, H.Y., Lin, Y.S.: Effects of water solutions on extracting green tea leaves. Sci. World J. 2013, 1–6 (2013)
Inti Ismail, N.N., Sapina Abdullah, U.U.: Determination of phenolic and antioxidant properties in tea and spent tea under various extraction method and determination of catechins, caffeine and gallic acid by HPLC. Int. J. Adv. Sci. Eng. Inf. Technol. 5, 158–164 (2015)
Xia, T., Shi, S., Wan, X.: Impact of ultrasonic-assisted extraction on the chemical and sensory quality of tea infusion. J. Food Eng. 74, 557–560 (2006)
Soltani, E., Ghaderi-Far, F., Baskin, C.C., Baskin, J.M.: Problems with using mean germination time to calculate rate of seed germination. Aust. J. Bot. 63, 631–635 (2015)
Espanany, A., Fallah, S., Tadayyon, A.: Seed priming improves seed germination and reduces oxidative stress in black cumin (Nigella sativa) in presence of cadmium. Ind. Crop Prod. 79, 195–204 (2016)
Silva, C.B., Marcos-Filho, J., Jourdan, P., Bennett, M.A.: Performance of bell pepper seeds in response to drum priming with addition of 24-epibrassinolide. HortScience 50, 873–878 (2015)
Lanteri, S., Saracco, F., Kraak, H.L., Bino, R.J.: The effects of priming on nuclear replication activity and germination of pepper (Capsicum annuum) and tomato (Lycopersicon esculentum) seeds. Seed Sci. Res. 4, 81–87 (1994)
Mahajan, G., Sarlach, R.S., Japinder, S., Gill, M.S.: Seed priming effects on germination, growth and yield of dry direct-seeded rice. J. Crop Improv. 25, 409–417 (2011)
Kubala, S., Garnczarska, M., Wojtyla, Ł., Clippe, A., Kosmala, A., Żmieńko, A., Lutts, S., Quinet, M.: Deciphering priminginduced improvement of rapeseed (Brassica napus L.) germination through an integrated transcriptomic and proteomic approach. Plant Sci. 231, 94–113 (2015)
Varier, A., Vari, A.K., Dadlani, M.: The sub cellular basis of seed priming. Curr. Sci. 99, 450–456 (2010)
Di Girolamo, G., Barbanti, L.: Treatment conditions and biochemical processes influencing seed priming effectiveness. Ital. J. Agron. 7, 178–188 (2012)
Sivritepe, H.O., Dourado, A.M.: The effect of priming treatments on the viability and accumulation of chromosomal damage in aged pea seeds. Ann. Bot. 75, 165–171 (1995)
Manonmani, V., Begum, M.A.J., Jayanthi, M.: Halo priming of seeds. Res. J. Seed Sci. 7, 1–13 (2014)
Sharma, S.N., Maheshwari, A.: Expression patterns of DNA repair genes associated with priming small and large chickpea (Cicer arietinum) seeds. Seed Sci. Technol. 43, 250–261 (2015)
Sedghi, M., Amanpour-Balaneji, B., Bakhshi, J.: Physiological enhancement of medicinal pumpkin seeds (Cucurbita pepo: var. styriaca) with different priming methods. Iran. J. Plant Physiol. 5, 1209–1215 (2014)
Nawaz, A., Amjad, M., Jahangir, M.M., Khan, S.M., Cui, H., Hu, J.: Induction of salt tolerance in tomato (Lycopersicon esculentum Mill:) seeds through sand priming. Aust. J. Crop Sci. 6, 1199–1203 (2012)
Younesi, O., Moradi, A.: Effect of priming of seeds of Medicago sativa ‘bami’ with gibberellic acid on germination, seedlings growth and antioxidant enzymes activity under salinity stress. J. Hortic. Res. 22, 167–174 (2015)
Afzal, I., Rauf, S., Basra, S.M., Murtaza, G.: Halopriming improves vigour, metabolism of reserves and ionic contents in wheat seedlings under salt stress. Plant Soil Environ. 54, 382–388 (2008)
Bakht, J., Shafi, M., Jamal, Y., Sher, H.: Response of maize (Zea mays L.) to seed priming with NaCl and salinity stress. Span. J. Agric. Res. 9, 252–261 (2011)
Saeed, R., Shah, P., Mirbahar, A.A., Jahan, B., Ahmed, N., Azeem, M., Ahmad, R.: Tea [Camellia sinensis (L.) Kuntze] leaf compost ameliorates the adverse effects of salinity on growth of cluster beans (Cyamopsis tetragonoloba L.). Pak. J. Bot. 48, 495–501 (2016)
Gomes, T., Pereira, J.A., Ramalhosa, E., Casal, S. & Baptista, P.: Effect of fresh and composted spent coffee grounds on lettuce growth, photosynthetic pigments and mineral composition, VII Congreso Iberico de Agroingenieria y Ciencias Horticolas, Madrid, 26–29 Agosto (2013)
Chrysargyris, A., Antoniou, O., Xylia, P., Petropoulos, S., Tzortzakis, N.: The use of spent coffee grounds in growing media for the production of Brassica seedlings in nurseries. Environ. Sci. Pollut. Res. (2020). https://doi.org/10.1007/s11356-020-07944-9
Sliwinska, E.: Nuclear DNA replication and seed quality. Seed Sci. Res. 19, 15–25 (2009)
Nadiah, N.I., Uthumporn, U.: Determination of phenolic and antioxidant properties in tea and spent tea under various extraction method and determination of catechins, caffeine and gallic acid by HPLC. Int. J. Adv. Sci. Eng. Inf. Technol. 5, 158–164 (2015)
Ballesteros, L.F., Teixeira, J.A., Mussatto, S.I.: Chemical, functional: and structural properties of spent coffee grounds and coffee silverskin. Food Bioprocess. Technol. 7, 3493–3503 (2014)
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gammoudi, N., Nagaz, K. & Ferchichi, A. Potential Use of Spent Coffee Grounds and Spent Tea Leaves Extracts in Priming Treatment to Promote In Vitro Early Growth of Salt-and Drought-Stressed Seedlings of Capsicum annuum L.. Waste Biomass Valor 12, 3341–3353 (2021). https://doi.org/10.1007/s12649-020-01216-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-020-01216-w