Abstract
Purpose
Tobacco stalk contained large amount of lignin, which is resistant to microbial degradation and limit the composting efficiency during composting. The purpose of this study is to enhance the lignin degradation efficiency, and consequently improve the composting efficiency and product quality.
Methods
Lignin degrading microorganisms (LDMs) were screened and inoculated to tobacco stalk compost, lignin degradation enzyme activities and lignin degradation rate were measured to evaluate the lignin degradation efficiency, other physico-chemical characters were determined as well to evaluate the composting efficiency and product quality.
Results
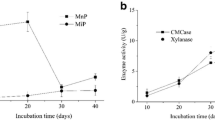
Two white-rot fungi Trametes hirsuta S13 and Pleurotus ostreatus S18 were screened as LDM according to their high lignin degradation efficiency. After inoculation, lignin peroxidase, manganese peroxidase and laccase were raised from (U g−1) 105.9, 372.9 and 460.68 to 374.7, 1095.6 and 1420.8, respectively. Consequently, lignin degradation rate increased nearly 2-fold (from 23.7 to 41.1%). In addition, organic matter degradation rate was increased from 26.5 to 31.2% with the addition of fungal inoculants. Degree of polymerization, humification index and germination index were also elevated, from 2.0, 17.8% and 108.3% to 3.5, 24.2% and 123.8%, respectively.
Conclusions
In this study, T. hirsuta S13 and P. ostreatus S18 were used as LDM and inoculated to tobacco stalk composting. After inoculation, the lignin degradation rate was increased, with the composting efficiency and the quality of composting product was improved as well.
Graphical Abstract







Similar content being viewed by others
References
Bernal, M.P., Alburquerque, J.A., Moral, R.: Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour. Technol. 100(22), 5444–5453 (2009). https://doi.org/10.1016/j.biortech.2008.11.027
Zhang, L., Zeng, G., Dong, H., Chen, Y., Zhang, J., Yan, M., Zhu, Y., Yuan, Y., Xie, Y., Huang, Z.: The impact of silver nanoparticles on the co-composting of sewage sludge and agricultural waste: evolutions of organic matter and nitrogen. Bioresour. Technol. 230, 132–139 (2017). https://doi.org/10.1016/j.biortech.2017.01.032
Zeng, G., Zhang, L., Dong, H., Chen, Y., Zhang, J., Zhu, Y., Yuan, Y., Xie, Y., Fang, W.: Pathway and mechanism of nitrogen transformation during composting: functional enzymes and genes under different concentrations of PVP-AgNPs. Bioresour. Technol. 253, 112–120 (2018). https://doi.org/10.1016/j.biortech.2017.12.095
Zhang, L., Zhang, J., Zeng, G., Dong, H., Chen, Y., Huang, C., Zhu, Y., Xu, R., Cheng, Y., Hou, K., Cao, W., Fang, W.: Multivariate relationships between microbial communities and environmental variables during co-composting of sewage sludge and agricultural waste in the presence of PVP-AgNPs. Bioresour. Technol. 261, 10–18 (2018). https://doi.org/10.1016/j.biortech.2018.03.089
Briški, F., Horgas, N., Vuković, M., Gomzi, Z.: Aerobic composting of tobacco industry solid waste—simulation of the process. Clean Technol. Environ. Policy 5(3–4), 295–301 (2003). https://doi.org/10.1007/s10098-003-0218-7
Jurado, M.M., Suarez-Estrella, F., Lopez, M.J., Vargas-Garcia, M.C., Lopez-Gonzalez, J.A., Moreno, J.: Enhanced turnover of organic matter fractions by microbial stimulation during lignocellulosic waste composting. Bioresour. Technol. 186, 15–24 (2015). https://doi.org/10.1016/j.biortech.2015.03.059
Liu, N., Zhou, J., Han, L., Huang, G.: Characterization of lignocellulosic compositions’ degradation during chicken manure composting with added biochar by phospholipid fatty acid (PLFA) and correlation analysis. Sci. Total Environ. 586, 1003–1011 (2017). https://doi.org/10.1016/j.scitotenv.2017.02.081
Wang, H.Y., Fan, B.Q., Hu, Q.X., Yin, Z.W.: Effect of inoculation with Penicillium expansum on the microbial community and maturity of compost. Bioresour. Technol. 102(24), 11189–11193 (2011). https://doi.org/10.1016/j.biortech.2011.07.044
Xu, J., Xu, X., Liu, Y., Li, H., Liu, H.: Effect of microbiological inoculants DN-1 on lignocellulose degradation during co-composting of cattle manure with rice straw monitored by FTIR and SEM. Environ. Prog. Sustain. Energy 35(2), 345–351 (2016). https://doi.org/10.1002/ep.12222
Tuomela, M., Vikman, M., Hatakka, A., Itävaara, M.: Biodegradation of lignin in a compost environment: a review. Bioresour. Technol. 72(2), 169–183 (2000). https://doi.org/10.1016/S0960-8524(99)00104-2
Leonowicz, A., Matuszewska, A., Luterek, J., Ziegenhagen, D., Wojtaś-Wasilewska, M., Cho, N.-S., Hofrichter, M., Rogalski, J.: Biodegradation of lignin by white rot fungi. Fungal Genet. Biol. 27(2), 175–185 (1999). https://doi.org/10.1006/fgbi.1999.1150
Zhang, C., Liu, L., Zeng, G.-M., Huang, D.-L., Lai, C., Huang, C., Wei, Z., Li, N.-J., Xu, P., Cheng, M., Li, F.-L., He, X., Lai, M., He, Y.: Utilization of nano-gold tracing technique: study the adsorption and transmission of laccase in mediator-involved enzymatic degradation of lignin during solid-state fermentation. Biochem. Eng. J. 91, 149–156 (2014). https://doi.org/10.1016/j.bej.2014.08.009
Archibald, F.S.: A new assay for lignin-type peroxidases employing the dye azure B. Appl. Environ. Microbiol. 58(9), 3110 (1992)
Poojary, H., Hoskeri, A., Kaur, A., Mugeraya, G.: Comparative production of ligninolytic enzymes from novel isolates of Basidiomycetes and their potential to degrade textile dyes. Nat. Sci. 10(10), 90–96 (2012)
Nishida, T.: Lignin biodegradation by wood-rotting fungi. I. Screening of lignin degrading fungi. Mokuzai Gakkaishi 34, 530–536 (1988)
Wei, X., Deng, X., Cai, D., Ji, Z., Wang, C., Yu, J., Li, J., Chen, S.: Decreased tobacco-specific nitrosamines by microbial treatment with Bacillus amyloliquefaciens DA9 during the air-curing process of burley tobacco. J. Agric. Food Chem. 62(52), 12701–12706 (2014). https://doi.org/10.1021/jf504084z
Tamura, K., Dudley, J., Nei, M., Kumar, S.: MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24(8), 1596–1599 (2007). https://doi.org/10.1093/molbev/msm092
Xin, Y., Huang, J., Deng, M., Zhang, W.: Culture-independent nested PCR method reveals high diversity of actinobacteria associated with the marine sponges Hymeniacidon perleve and Sponge sp. Antonie Van Leeuwenhoek 94(4), 533–542 (2008). https://doi.org/10.1007/s10482-008-9270-y
Lopez, M.J., del Vargas-García, M.C., Suárez-Estrella, F., Nichols, N.N., Dien, B.S., Moreno, J.: Lignocellulose-degrading enzymes produced by the ascomycete Coniochaeta ligniaria and related species: application for a lignocellulosic substrate treatment. Enzyme Microb. Technol. 40(4), 794–800 (2007). https://doi.org/10.1016/j.enzmictec.2006.06.012
Zeng, G.M., Huang, H.L., Huang, D.L., Yuan, X.Z., Jiang, R.Q., Yu, M., Yu, H.Y., Zhang, J.C., Wang, R.Y., Liu, X.L.: Effect of inoculating white-rot fungus during different phases on the compost maturity of agricultural wastes. Process Biochem. 44(4), 396–400 (2009). https://doi.org/10.1016/j.procbio.2008.11.012
Tiquia, S.M., Tam, N.F.Y., Hodgkiss, I.J.: Effects of composting on phytotoxicity of spent pig-manure sawdust litter. Environ. Pollut. 93(3), 249–256 (1996). https://doi.org/10.1016/S0269-7491(96)00052-8
Pollegioni, L., Tonin, F., Rosini, E.: Lignin-degrading enzymes. FEBS J. 282(7), 1190–1213 (2015). https://doi.org/10.1111/febs.13224
Tiquia, S.M., Tam, N.F.Y.: Elimination of phytotoxicity during co-composting of spent pig-manure sawdust litter and pig sludge. Bioresour. Technol. 65(1), 43–49 (1998). https://doi.org/10.1016/S0960-8524(98)00024-8
Tang, J.-C., Shibata, A., Zhou, Q., Katayama, A.: Effect of temperature on reaction rate and microbial community in composting of cattle manure with rice straw. J. Biosci. Bioeng. 104(4), 321–328 (2007). https://doi.org/10.1263/jbb.104.321
Kopcic, N., Vukovic Domanovac, M., Kucic, D., Briski, F.: Evaluation of laboratory-scale in-vessel co-composting of tobacco and apple waste. Waste Manag. 34(2), 323–328 (2014). https://doi.org/10.1016/j.wasman.2013.11.001
Šnajdr, J., Baldrian, P.: Temperature affects the production, activity and stability of ligninolytic enzymes in Pleurotus ostreatus and Trametes versicolor. Folia Microbiol. 52(5), 498–502 (2007). https://doi.org/10.1007/bf02932110
dos Santos, T.C., de Brito, A.R., Bonomo, R.C.F., Pires, A.J.V., Aguiar-Oliveira, E., Silva, T.P., Franco, M.: Statistical optimization of culture conditions and characterization for ligninolytic enzymes produced from Rhizopus sp. using prickly palm cactus husk. Chem. Eng. Commun. 204(1), 55–63 (2017). https://doi.org/10.1080/00986445.2016.1230851
Nakhshiniev, B., Biddinika, M.K., Gonzales, H.B., Sumida, H., Yoshikawa, K.: Evaluation of hydrothermal treatment in enhancing rice straw compost stability and maturity. Bioresour. Technol. 151, 306–313 (2014). https://doi.org/10.1016/j.biortech.2013.10.083
Mahimairaja, S., Bolan, N.S., Hedley, M.J., Macgregor, A.N.: Losses and transformation of nitrogen during composting of poultry manure with different amendments: an incubation experiment. Bioresour. Technol. 47(3), 265–273 (1994). https://doi.org/10.1016/0960-8524(94)90190-2
Riffaldi, R., Levi-Minzi, R., Pera, A., de Bertoldi, M.: Evaluation of compost maturity by means of chemical and microbial analyses. Waste Manag. Res. 4(4), 387–396 (1986)
Zhang, L., Sun, X.: Changes in physical, chemical, and microbiological properties during the two-stage co-composting of green waste with spent mushroom compost and biochar. Bioresour. Technol. 171, 274–284 (2014). https://doi.org/10.1016/j.biortech.2014.08.079
Iglesias Jiménez, E., Pérez García, V.: Determination of maturity indices for city refuse composts. Agric. Ecosyst. Environ. 38(4), 331–343 (1992). https://doi.org/10.1016/0167-8809(92)90154-4
Tang, J.-C., Maie, N., Tada, Y., Katayama, A.: Characterization of the maturing process of cattle manure compost. Process Biochem. 41(2), 380–389 (2006). https://doi.org/10.1016/j.procbio.2005.06.022
Aggelis, G., Ehaliotis, C., Nerud, F., Stoychev, I., Lyberatos, G., Zervakis, G.I.: Evaluation of white-rot fungi for detoxification and decolorization of effluents from the green olive debittering process. Appl. Microbiol. Biotechnol. 59(2–3), 353–360 (2002). https://doi.org/10.1007/s00253-002-1005-9
Acknowledgements
This work was supported by the National Program on Key Basic Research Project (973 Program, No. 2015CB150505), the Science and Technology Program of Wuhan (20160201010086), and the Key Project of China National Tobacco Corporation Hubei Company.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, L., Yuan, H., Yang, Y. et al. Enhanced Lignin Degradation in Tobacco Stalk Composting with Inoculation of White-Rot Fungi Trametes hirsuta and Pleurotus ostreatus. Waste Biomass Valor 11, 3525–3535 (2020). https://doi.org/10.1007/s12649-019-00692-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-019-00692-z