Abstract
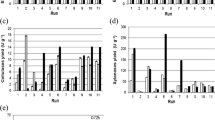
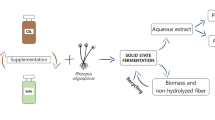
In this work four agroindustrial cakes were used as raw material both for production of enzyme pools containing amylases and accessory hydrolases by solid-state fermentation (SSF) and for cold starch hydrolysis. Eight fungal strains from the genera Aspergillus and Penicillium were screened for enzyme production, and their enzyme extracts were then evaluated in the hydrolysis of raw cakes. Babassu cake was the most suitable raw material for endoamylases, exoamylases and proteases production. The highest activities of these three enzymes were produced by A. awamori IOC-3914 (29.8 U g−1), A. wentii (47.8 U g−1) and P. verrucosum (27.5 U g−1), respectively. Regarding cellulases and xylanases, maximum activities (12.8 and 598.0 U g−1, respectively) were obtained by A. awamori IOC-3915 in castor seed residue. Saccharification studies showed a flexible applicability of the raw extracts to hydrolyze different cakes. A maximum total reducing sugars concentration of 13.9 g L−1 was obtained from babassu cake using a raw enzyme extract produced by A. awamori IOC-3915, without any concentration or purification steps. The present results demonstrate that a low-cost SSF process can supply enzyme extracts with a high potential for application in the cold hydrolysis of raw starch from agroindustrial cakes.




Similar content being viewed by others
References
Godfrey, T., West, S.: Industrial Enzymology. The MacMillan Press Ltd, London (1996)
Gupta, R., Gigras, P., Mohapatra, H., Goswami, V.K., Chauhan, B.: Microbial α-amylases: a biotechnological perspective. Proc. Biochem. 38, 1599–1616 (2003)
Norouzian, D., Akbarzadeh, A., Scharer, J.M., Young, M.M.: Fungal glucoamylases. Biotechnol. Adv. 24, 80–85 (2006)
Van der Maarel, M.J.E.C., van der Veen, B., Uitdehaag, J.C.M., Leemhuis, H., Dijkhuizen, L.: Properties and applications of starch-converting enzymes of the α-amylase family. J. Biotechnol. 94, 137–155 (2002)
Ramachandran, S., Patel, A.K., Nampoothiri, K.M., Chandran, S., Szakacs, G., Soccol, C.R., Pandey, A.: Alpha amylase from a fungal culture grown on oil cakes and its properties. Braz. Arch. Biol. Technol. 47, 309–317 (2004)
Faostat : http://faostat.fao.org. Accessed in: March 28th, 2011
Gombert, A.K., Pinto, A.L., Castilho, L.R., Freire, D.M.G.: Lipase production by Penicillium restrictum in solid state fermentation using babassu oil cake as substrate. Proc. Biochem. 35, 85–90 (1999)
Baruque Filho, E.A., Baruque, M.G.A., Sant’Anna Jr, G.L.: Babassu coconut starch liquefaction: an industrial scale approach to improve conversion yield. Bioresour. Technol. 75, 49–55 (2000)
Kunamneni, A., Permaul, K., Singh, S.: Amylase production in solid state fermentation by the thermophilic fungus Thermomyces lanuginosus. J. Biosci. Bioeng. 100, 168–171 (2005)
Vidal Jr, B.C., Rausch, K.D., Tumbleson, M.E., Singh, V.: Protease treatment to improve ethanol fermentation in modified dry grind corn process. Cereal Chem. 86, 323–328 (2009)
Wang, P., Johnston, D.B., Rausch, K.D., Schmidt, S.J., Tumbleson, M.E., Singh, V.: Effects of protease and urea on a granular starch hydrolyzing process for corn ethanol production. Cereal Chem. 86, 319–322 (2009)
Kim, Y., Mosier, N., Ladisch, M.R.: Process simulation of modified dry grind ethanol plant with recycle of pretreated and enzymatically hydrolyzed distillers’ grains. Bioresour. Technol. 99, 5177–5192 (2008)
Polaina, J., MacCabe, A.P.: Industrial Enzymes: Structure, Functions and Applications. Springer, Dordrecht (2007)
Berven, D.: The making of broin Project X. Ethanol Prod. Mag. 11, 67–71 (2005)
Galvez, A.: Analyzing cold enzyme starch hydrolysis technology in new ethanol plant design. Ethanol Prod. Mag. 11, 58–60 (2005)
Robertson, G.H., Wong, D.W.S., Lee, C.C., Wagschal, K., Smith, M.R., Orts, W.J.: Native or raw starch digestion: a key step in energy efficient biorefining of grain. J. Agri. Food Chem. 54, 353–365 (2006)
Gibreel, A., Sandercock, J.R., Lan, J., Goonewardene, L.A., Zijlstra, R.T., Curtis, J.M., Bressler, D.C.: Fermentation of barley by using Saccharomyces cerevisiae: examination of barley as a feedstock for bioethanol production and value-added products. Appl. Environ. Microbiol. 75, 1363–1372 (2009)
Matsubara, T., Ammar, Y.B., Anindyawati, T., Yamamoto, S., Ito, K., Iizuka, M., Minamiura, N.: Degradation of raw starch granules by α-amylase purified from culture of Aspergillus awamori KT-11. J. Biochem. Mol. Biol. 37, 422–428 (2004)
Ertan, F., Yagar, H., Balkan, B.: Some properties of free and immobilized α-amylase from Penicillium griseofulvum by solid-state fermentation. Prep. Biochem. Biotechnol. 36, 81–91 (2006)
Castro, A.M., Carvalho, M.L.A., Leite, S.G.F., Pereira Jr, N.: Cellulases from Penicillium funiculosum: production, properties and application to cellulose hydrolysis. J. Ind. Microbiol. Biotechnol. 37, 151–158 (2010)
De Vries, R., Visser, J.: Aspergillus enzymes involved in degradation of plant cell wall polysaccharides. Microbiol. Mol. Biol. Rev. 65, 497–522 (2001)
Ghose, T.K.: Measurement of cellulase activities. Pure Appl. Chem. 59, 257–268 (1987)
Olajuyigbe, F.M.: Influence of media composition on acid protease production by Aspergillus niger (NRRL-1785). Global J. Pure. Appl. Sci. 12, 85–88 (2006)
Khalil, C.N., Leite, L.: Process for producing biodiesel fuel using triglyceride-rich oleaginous seed directly in a transesterification reaction in the presence of an alkaline alkoxide catalyst. US Patent 7112229 (2006)
AOAC: AOAC Official Method 996.11: Starch (Total) in Cereal Products. Official Methods of Analysis of the AOAC International, 16th Ed. Supplement 1998. AOAC, Washington (1995)
Britton, H.T.S., Robinson, R.E.: Universal buffer solutions and the dissociation constant of veronal. J. Chem. Soc. 1931, 1456–1462 (1931)
Fernandes, L.P., Ulhoa, C.J., Asquieri, E.R., Monteiro, V.N.: Produção de amilases pelo fungo Macrophomina phaseolina. Revista Eletrônica de Farmácia IV, 43–51 (2007)
Figueira, E.L.Z., Hirooka, E.Y.: Culture medium for amylase production by toxigenic fungi. Braz. Arch. Biol. Technol. 43, 461–467 (2000)
Riaz, M., Perveen, R., Javed, M.R., Nadeem, H., Rashid, M.H.: Kinetic and thermodynamic properties of novel glucoamylase from Humicola sp. Enzyme Microb. Technol. 41, 558–564 (2007)
Bendicho, S., Martí, G., Hernández, T., Martín, O.: Determination of proteolytic activity in different milk systems. Food Chem. 79, 245–249 (2002)
Sumner, J.B.: Dinitrosalicylic acid: a reagent for the estimation of sugar in normal and diabetic urine. J. Biol. Chem. 47, 5–9 (1921)
Alva, S., Anupama, J., Savla, J., Chiu, Y.Y., Vyshali, P., Shruti, M., Yogeetha, B.S., Bhavya, D., Purvi, J., Ruchi, K., Kumudini, B.S., Varalakshmi, K.N.: Production and characterization of fungal amylase enzyme isolated from Aspergillus sp. JGI12 in solid state culture. Afr. J. Biotechnol. 6, 576–581 (2007)
Costa, J.A.V., Colla, E., Magagnin, G., Santos, L.O., Vendruscolo, M., Bertolin, T.E.: Simultaneous amyloglucosidase and exo-polygalacturonase production by Aspergillus niger using solid-state fermentation. Braz. Arch. Biol. Technol. 50, 759–766 (2007)
Pandey, A., Nigam, P., Soccol, C.R., Soccol, V.T., Singh, D., Mohan, R.: Advances in microbial amylases. Biotechnol. Appl. Biochem. 31, 135–152 (2000)
Prakasham, R.S., Rao, C.S., Rao, R.S., Sarma, P.N.: Enhancement of acid amylase production by an isolated Aspergillus awamori. J. Appl. Microbiol. 102, 204–211 (2007)
Sauer, J., Sigurskjold, B.W., Christensen, U., Frandsen, T.P., Mirgorodskaya, E., Harrison, M., Roepstorff, P., Svensson, B.: Glucoamylase: structure/function relationships, and protein engineering. Biochim. Biophys. Acta 1543, 275–293 (2000)
Kempka, A.P., Lipke, N.L., Pinheiro, T.L.F., Menoncin, S., Treichel, H., Freire, D.M.G., Di Luccio, M., Oliveira, D.: Response surface method to optimize the production and characterization of lipase from Penicillium verrucosum in solid-state fermentation. Bioprocess Biosyst. Eng. 31, 119–125 (2008)
Viniegra-González, G., Favela-Torres, E., Aguilar, C.N., Romero-Gomez, S.J., Diaz-Godínez, G., Augur, C.: Advantages of fungal enzyme production in solid state over liquid fermentation systems. Biochem. Eng. J. 13, 157–167 (2003)
Mitchell, D.A., von Meien, O.F., Krieger, N.: Recent developments in modeling of solid-state fermentation: heat and mass transfer in bioreactors. Biochem. Eng. J 13, 137–147 (2003)
Müller, C., McIntyre, M., Hansen, K., Nielsen, J.: Metabolic engineering of the morphology of Aspergillus oryzae by altering chitin synthesis. Appl. Environ. Microbiol. 68, 1827–1836 (2002)
Gutarra, M.L.E., Godoy, M.G., Silva, J.N., Guedes, I.A., Lins, U., Castilho, L.R., Freire, D.M.G.: Lipase production and Penicillium simplicissimum morphology in solid-state and submerged fermentations. Biotechnol. J. 4, 1450–1459 (2009)
Hölker, U., Lenz, J.: Solid-state fermentation—are there any biotechnological advantages? Curr. Opin. Microbiol. 8, 301–306 (2005)
Rajagopalan, S., Modak, J.M.: Evaluation of relative growth limitation due to depletion of glucose and oxygen during fungal growth on a spherical solid particle. Chem. Eng. Sci. 50, 803–811 (1995)
Vidal Jr, B.C., Rausch, K.D., Tumbleson, M.E., Singh, V.: Kinetics of granular starch hydrolysis in corn dry-grind process. Starch/Stärke 61, 448–456 (2009)
Balan, V., Rogers C.A., Chundawat, S.P.S., Sousa, L.C., Slininger, P.J., Gupta, R., Dale, B.E.: Conversion of extracted oil cake fibers into bioethanol including DDGS, canola, sunflower, sesame, soy, and peanut for integrated biodiesel processing. J. Am. Oil Chem. Soc. 86, 157–165 (2009)
Zhang, Y.H.P., Lynd, L.R.: Toward an aggregated understanding of enzymatic hydrolysis of cellulose: noncomplexed cellulose systems. Biotechnol. Bioeng. 88, 797–824 (2004)
Castro, A.M., Pereira Jr, N.: Production, properties and application of cellulases in the hydrolysis of agroindustrial residues. Quim. Nova 33, 181–188 (2010)
Lang, X., Hill, G.A., MacDonald, D.G.: Recycle bioreactor for bioethanol production from wheat starch I. Cold enzyme hydrolysis. Energy Sour 23, 417–425 (2001)
Sarikaya, E., Higasa, T., Adachi, M., Mikami, B.: Comparison of degradation abilities of α- and β-amylases on raw starch granules. Proc. Biochem. 35, 711–715 (2000)
Textor, S.D., Hill, G.A., MacDonald, D.G., Denis, E.S.T.: Cold enzyme hydrolysis of wheat starch granules. Can. J. Chem. Eng. 76, 87–93 (1998)
Faulds, C.B., Robertson, J.A., Waldron, K.W.: Effect of pH on solubilization of brewer’s spent grain by microbial carbohydrases and proteases. J. Agric. Food Chem. 56, 7038–7043 (2008)
Melo, W.C., Santos, A.S., Santa Anna, L.M.M., Pereira Jr, N.: Acid and enzymatic hydrolysis of the residue from castor seed (Ricinus communis L.) oil extraction for ethanol production: detoxification and biodiesel process integration. J. Braz. Chem. Soc. 19, 418–425 (2008)
Castro, A.M., Carvalho, D.F., Freire, D.M.G., Castilho, L.R.: Economic analysis of the production of amylases and other hydrolases by Aspergillus awamori in solid-state fermentation of babassu cake. Enzyme Res. 2010. Article ID 576872. doi:10.4061/2010/576872
Castilho, L.R., Polato, C.M.S., Baruque, E.A., Sant’Anna Jr, G.L., Freire, D.M.G.: Economic analysis of lipase production by Penicillium restrictum in solid-state and submerged fermentations. Biochem. Eng. J. 4, 239–247 (2000)
Castro, A.M., Andrea, T.V., Castilho, L.R., Freire, D.M.G.: Use of mesophilic fungal amylases produced by solid-state fermentation in the cold hydrolysis of raw babassu cake starch. Appl. Biochem. Biotechnol. 162, 1612–1625 (2010)
Acknowledgments
The authors wish to thank Dr. Edmond Baruque (Tocantis Babaçu S.A.) for kindly providing babassu cake and Ms. Mariana Paixão (Membrane Separation Processes and Polymer Laboratory, PAM/COPPE, Federal University of Rio de Janeiro) for her technical assistance in SEM analyses. The authors also gratefully acknowledge the financial support from CNPq, FAPERJ and ANP/PETROBRAS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Castro, A.M., de Andréa, T.V., Carvalho, D.F. et al. Valorization of Residual Agroindustrial Cakes by Fungal Production of Multienzyme Complexes and Their Use in Cold Hydrolysis of Raw Starch. Waste Biomass Valor 2, 291–302 (2011). https://doi.org/10.1007/s12649-011-9075-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-011-9075-5