Abstract
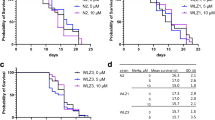
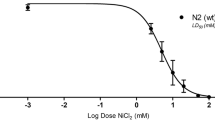
Methylmercury (MeHg) neurotoxicity exhibits age-dependent effects with a latent and/or persistent neurotoxic effect on aged animals. Individual susceptibility to MeHg neurotoxicity is governed by both exposure duration and genetic factors that can magnify or mitigate the pathologic processes associated with this exposure. We previously showed the G2019S mutation of leucine-rich repeat kinase 2 (LRRK2) modulates the response of worms to high levels of MeHg, mitigating its effect on neuronal morphology in pre-vesicles in cephalic (CEP) dopaminergic neurons. Here we sought to better understand the long-term effects of MeHg exposure at low levels (100-fold lower than that in our previous report) and the modulatory role of the LRRK2 mutation. Worms exposed to MeHg (10 or 50 nM) at the larval stage (L1 stage) were compared at adult stages (young age: day 1 adult; middle age: day 5 adult; old age: day 10 adult) for the swimming speeds in M9 buffer, moving speeds during locomotion on an OP50-seeded plate, and the numbers of CEP dopaminergic pre-vesicles, vesicular structures originating from the dendrites of CEP for exportation of cellular content. In addition, the expression levels of Caenorhabditis elegans homologs of dopamine transporter (dat-1) and tyrosine hydroxylase (cat-2) were also analyzed at these adult stages. Our data showed that swimming speeds were reduced in wild-type worms at the day 10 adult stage at 50 nM MeHg level; yet, reduced swimming speeds were noted in the G2019S LRRK2 transgenic line upon MeHg exposures as low as 10 nM. Compared to locomotor speeds, swimming speeds appear to be more sensitive to the behavioral effects of developmental MeHg exposures, as the locomotor speeds were largely intact and indistinguishable from controls following MeHg exposures. Furthermore, we showed an age-dependent modulation of dat-1 and cat-2 expressions, which could also be modified by the LRRK2 mutation. Although MeHg exposures did not change the number of pre-vesicles, the LRRK2 mutation was associated with increased numbers of pre-vesicles in aged worms. Our data suggest that the latent behavioral effects of MeHg are sensitized by the G2019S LRRK2 mutation, and the underlying mechanism likely involves age-dependent changes in dopaminergic signaling.







Similar content being viewed by others
References
Andreoli V, Sprovieri F (2017) Genetic aspects of susceptibility to mercury toxicity: an overview. Int J Environ Res Public Health 14(1). https://doi.org/10.3390/ijerph14010093
Bakir F, Damluji SF, Amin-Zaki L, Murtadha M, Khalidi A, al-Rawi NY, Tikriti S, Dahahir HI, Clarkson TW, Smith JC, Doherty RA, (1973) Methylmercury poisoning in Iraq. Science 181(4096):230–241. https://doi.org/10.1126/science.181.4096.230
Ball N, Teo WP, Chandra S, Chapman J (2019) Parkinson’s disease and the environment. Front Neurol 10:218. https://doi.org/10.3389/fneur.2019.00218
Bannon MJ, Poosch MS, Xia Y, Goebel DJ, Cassin B, Kapatos G (1992) Dopamine transporter mRNA content in human substantia nigra decreases precipitously with age. Proc Natl Acad Sci U S A 89(15):7095–7099. https://doi.org/10.1073/pnas.89.15.7095
Bannon MJ, Whitty CJ (1997) Age-related and regional differences in dopamine transporter mRNA expression in human midbrain. Neurology 48(4):969–977. https://doi.org/10.1212/wnl.48.4.969
Bouhouche A, Tibar H, Ben El Haj R, El Bayad K, Razine R, Tazrout S, Skalli A, Bouslam N, Elouardi L, Benomar A, Yahyaoui M, Regragui W (2017) LRRK2 G2019S mutation: prevalence and clinical features in Moroccans with Parkinson’s disease. Parkinsons Dis 2017:2412486. https://doi.org/10.1155/2017/2412486
Bové J, Prou D, Perier C, Przedborski S (2005) Toxin-induced models of Parkinson’s disease. NeuroRx 2(3):484–494. https://doi.org/10.1602/neurorx.2.3.484
Clark LN, Wang Y, Karlins E, Saito L, Mejia-Santana H, Harris J, Louis ED, Cote LJ, Andrews H, Fahn S, Waters C, Ford B, Frucht S, Ottman R, Marder K (2006) Frequency of LRRK2 mutations in early- and late-onset Parkinson disease. Neurology 67(10):1786–1791. https://doi.org/10.1212/01.wnl.0000244345.49809.36
Colón-Rodríguez A, Hannon HE, Atchison WD (2017) Effects of methylmercury on spinal cord afferents and efferents-a review. Neurotoxicology 60:308–320. https://doi.org/10.1016/j.neuro.2016.12.007
Davis LE, Kornfeld M, Mooney HS, Fiedler KJ, Haaland KY, Orrison WW, Cernichiari E, Clarkson TW (1994) Methylmercury poisoning: long-term clinical, radiological, toxicological, and pathological studies of an affected family. Ann Neurol 35(6):680–688. https://doi.org/10.1002/ana.410350608
Dhondt I, Verschuuren C, Zečić A, Loier T, Braeckman BP, De Vos WH (2021) Prediction of biological age by morphological staging of sarcopenia in Caenorhabditis elegans. Dis Model Mech 14(11). https://doi.org/10.1242/dmm.049169
Di Monte DA (2003) The environment and Parkinson’s disease: is the nigrostriatal system preferentially targeted by neurotoxins? Lancet Neurol 2(9):531–538. https://doi.org/10.1016/s1474-4422(03)00501-5
Droby A, Artzi M, Lerman H, Hutchison RM, Bashat DB, Omer N, Gurevich T, Orr-Urtreger A, Cohen B, Cedarbaum JM, Sapir EE, Giladi N, Mirelman A, Thaler A (2022) Aberrant dopamine transporter and functional connectivity patterns in LRRK2 and GBA mutation carriers. NPJ Parkinsons Dis 8(1):20. https://doi.org/10.1038/s41531-022-00285-z
Ekino S, Susa M, Ninomiya T, Imamura K, Kitamura T (2007) Minamata disease revisited: an update on the acute and chronic manifestations of methyl mercury poisoning. J Neurol Sci 262(1–2):131–144. https://doi.org/10.1016/j.jns.2007.06.036
Eto K, Marumoto M, Takeya M (2010) The pathology of methylmercury poisoning (Minamata disease): the 50th Anniversary of Japanese Society of Neuropathology. Neuropathology 30(5):471–479. https://doi.org/10.1111/j.1440-1789.2010.01119.x
Evans HL, Garman RH, Weiss B (1977) Methylmercury: exposure duration and regional distribution as determinants of neurotoxicity in nonhuman primates. Toxicol Appl Pharmacol 41(1):15–33. https://doi.org/10.1016/0041-008x(77)90051-5
Faro LR, do Nascimento JL, Alfonso M, Durán R (2002) Mechanism of action of methylmercury on in vivo striatal dopamine release. Possible involvement of dopamine transporter. Neurochem Int 40(5):455–465. https://doi.org/10.1016/s0197-0186(01)00098-5
Ghosh DD, Sanders T, Hong S, McCurdy LY, Chase DL, Cohen N, Koelle MR, Nitabach MN (2016) Neural architecture of hunger-dependent multisensory decision making in C. elegans. Neuron 92(5):1049–1062. https://doi.org/10.1016/j.neuron.2016.10.030
Goldwurm S, Di Fonzo A, Simons EJ, Rohé CF, Zini M, Canesi M, Tesei S, Zecchinelli A, Antonini A, Mariani C, Meucci N, Sacilotto G, Sironi F, Salani G, Ferreira J, Chien HF, Fabrizio E, Vanacore N, Dalla Libera A, Stocchi F, Diroma C, Lamberti P, Sampaio C, Meco G, Barbosa E, Bertoli-Avella AM, Breedveld GJ, Oostra BA, Pezzoli G, Bonifati V (2005) The G6055A (G2019S) mutation in LRRK2 is frequent in both early and late onset Parkinson’s disease and originates from a common ancestor. J Med Genet 42(11):e65. https://doi.org/10.1136/jmg.2005.035568
Gourgou E, Adiga K, Goettemoeller A, Chen C, Hsu AL (2021) Caenorhabditis elegans learning in a structured maze is a multisensory behavior. iScience 24(4):102284. https://doi.org/10.1016/j.isci.2021.102284
Gupta M, Stoler MH, Fossom LH, Tank AW (1990) Tyrosine hydroxylase mRNA in the dopaminergic neurons of young adult and aged mice by in situ hybridization. Neurosci Lett 119(1):49–52. https://doi.org/10.1016/0304-3940(90)90752-u
Harada M (1995) Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol 25(1):1–24. https://doi.org/10.3109/10408449509089885
Harada M, Akagi H, Tsuda T, Kizaki T, Ohno H (1999) Methylmercury level in umbilical cords from patients with congenital Minamata disease. Sci Total Environ 234(1–3):59–62. https://doi.org/10.1016/s0048-9697(99)00255-7
Healy DG, Falchi M, O’Sullivan SS, Bonifati V, Durr A, Bressman S, Brice A, Aasly J, Zabetian CP, Goldwurm S, Ferreira JJ, Tolosa E, Kay DM, Klein C, Williams DR, Marras C, Lang AE, Wszolek ZK, Berciano J, Schapira AH, Lynch T, Bhatia KP, Gasser T, Lees AJ, Wood NW (2008) Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: a case-control study. Lancet Neurol 7(7):583–590. https://doi.org/10.1016/s1474-4422(08)70117-0
Karagas MR, Choi AL, Oken E, Horvat M, Schoeny R, Kamai E, Cowell W, Grandjean P, Korrick S (2012) Evidence on the human health effects of low-level methylmercury exposure. Environ Health Perspect 120(6):799–806. https://doi.org/10.1289/ehp.1104494
Ke T, Aschner MJN (2019) Bacteria Affect Caenorhabditis Elegans Responses to MeHg Toxicity 75:129–135
Ke T, Gonçalves FM, Gonçalves CL, Dos Santos AA, Rocha JBT, Farina M, Skalny A, Tsatsakis A, Bowman AB, Aschner M (2019) Post-translational modifications in MeHg-induced neurotoxicity. Biochim Biophys Acta Mol Basis Dis 1865(8):2068-2081. https://doi.org/10.1016/j.bbadis.2018.10.024. Epub 2018 Oct 29. PMID: 30385410; PMCID: PMC6488462
Ke T, Prince LM, Bowman AB, Aschner M (2021a) Latent alterations in swimming behavior by developmental methylmercury exposure are modulated by the homolog of tyrosine hydroxylase in Caenorhabditis elegans. Neurotoxicol Teratol 85:106963. https://doi.org/10.1016/j.ntt.2021.106963
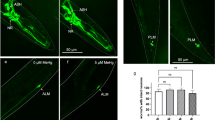
Ke T, Santamaria A, Rocha JBT, Tinkov A, Bornhorst J, Bowman AB, Aschner M (2020a) Cephalic neuronal vesicle formation is developmentally dependent and modified by methylmercury and sti-1 in Caenorhabditis elegans. Neurochem Res 45(12):2939–2948. https://doi.org/10.1007/s11064-020-03142-8
Ke T, Santamaria A, Rocha JBT, Tinkov AA, Lu R, Bowman AB, Aschner M (2020b) The role of human LRRK2 in methylmercury-induced inhibition of microvesicle formation of cephalic neurons in Caenorhabditis elegans. Neurotox Res 38(3):751–764. https://doi.org/10.1007/s12640-020-00262-5
Ke T, Tinkov AA, Skalny AV, Bowman AB, Rocha JBT, Santamaria A, Aschner M (2021b) Developmental exposure to methylmercury and ADHD, a literature review of epigenetic studies. Environ Epigenet 7(1):dvab014. https://doi.org/10.1093/eep/dvab014
Ke T, Tsatsakis A, Santamaria A, Antunes Soare FA, Tinkov AA, Docea AO, Skalny A, Bowman AB, Aschner M (2020c) Chronic exposure to methylmercury induces puncta formation in cephalic dopaminergic neurons in Caenorhabditis elegans. Neurotoxicology. https://doi.org/10.1016/j.neuro.2020.01.003
Kedzierski W, Porter JC (1990) Quantitative study of tyrosine hydroxylase mRNA in catecholaminergic neurons and adrenals during development and aging. Brain Res Mol Brain Res 7(1):45–51. https://doi.org/10.1016/0169-328x(90)90072-l
Kimura KD, Fujita K, Katsura I (2010) Enhancement of odor avoidance regulated by dopamine signaling in Caenorhabditis elegans. J Neurosci 30(48):16365–16375. https://doi.org/10.1523/jneurosci.6023-09.2010
Kindt KS, Quast KB, Giles AC, De S, Hendrey D, Nicastro I, Rankin CH, Schafer WR (2007) Dopamine mediates context-dependent modulation of sensory plasticity in C. elegans. Neuron 55(4):662–676. https://doi.org/10.1016/j.neuron.2007.07.023
Kouroupi G, Taoufik E, Vlachos IS, Tsioras K, Antoniou N, Papastefanaki F, Chroni-Tzartou D, Wrasidlo W, Bohl D, Stellas D, Politis PK, Vekrellis K, Papadimitriou D, Stefanis L, Bregestovski P, Hatzigeorgiou AG, Masliah E, Matsas R (2017) Defective synaptic connectivity and axonal neuropathology in a human iPSC-based model of familial Parkinson’s disease. Proc Natl Acad Sci U S A 114(18):E3679-e3688. https://doi.org/10.1073/pnas.1617259114
Lichtenberg M, Mansilla A, Zecchini VR, Fleming A, Rubinsztein DC (2011) The Parkinson’s disease protein LRRK2 impairs proteasome substrate clearance without affecting proteasome catalytic activity. Cell Death Dis 2(8):e196. https://doi.org/10.1038/cddis.2011.81
Lin H, Ascher DB, Myung Y, Lamborg CH, Hallam SJ, Gionfriddo CM, Holt KE, Moreau JW (2021) Mercury methylation by metabolically versatile and cosmopolitan marine bacteria. Isme j 15(6):1810–1825. https://doi.org/10.1038/s41396-020-00889-4
Longo F, Mercatelli D, Novello S, Arcuri L, Brugnoli A, Vincenzi F, Russo I, Berti G, Mabrouk OS, Kennedy RT, Shimshek DR, Varani K, Bubacco L, Greggio E, Morari M (2017) Age-dependent dopamine transporter dysfunction and Serine129 phospho-α-synuclein overload in G2019S LRRK2 mice. Acta Neuropathol Commun 5(1):22. https://doi.org/10.1186/s40478-017-0426-8
Lüth T, König IR, Grünewald A, Kasten M, Klein C, Hentati F, Farrer M, Trinh J (2020) Age at onset of LRRK2 p.Gly2019Ser is related to environmental and lifestyle factors. Mov Disord 35(10):1854–1858. https://doi.org/10.1002/mds.28238
McDonald PW, Hardie SL, Jessen TN, Carvelli L, Matthies DS, Blakely RD (2007) Vigorous motor activity in Caenorhabditis elegans requires efficient clearance of dopamine mediated by synaptic localization of the dopamine transporter DAT-1. J Neurosci 27(51):14216–14227. https://doi.org/10.1523/jneurosci.2992-07.2007
Melrose HL, Dächsel JC, Behrouz B, Lincoln SJ, Yue M, Hinkle KM, Kent CB, Korvatska E, Taylor JP, Witten L, Liang YQ, Beevers JE, Boules M, Dugger BN, Serna VA, Gaukhman A, Yu X, Castanedes-Casey M, Braithwaite AT, Ogholikhan S, Yu N, Bass D, Tyndall G, Schellenberg GD, Dickson DW, Janus C, Farrer MJ (2010) Impaired dopaminergic neurotransmission and microtubule-associated protein tau alterations in human LRRK2 transgenic mice. Neurobiol Dis 40(3):503–517. https://doi.org/10.1016/j.nbd.2010.07.010
Myers GJ, Davidson PW (1998) Prenatal methylmercury exposure and children: neurologic, developmental, and behavioral research. Environ Health Perspect 106 Suppl 3(Suppl 3):841–847. https://doi.org/10.1289/ehp.98106841
Nogara PA, Oliveira CS, Schmitz GL, Piquini PC, Farina M, Aschner M, Rocha JBT (2019) Methylmercury’s chemistry: From the environment to the mammalian brain. Biochim Biophys Acta Gen Subj 1863(12):129284. https://doi.org/10.1016/j.bbagen.2019.01.006
Omura DT, Clark DA, Samuel AD, Horvitz HR (2012) Dopamine signaling is essential for precise rates of locomotion by C. elegans. PLoS One 7(6):e38649. https://doi.org/10.1371/journal.pone.0038649
Orenstein SJ, Kuo SH, Tasset I, Arias E, Koga H, Fernandez-Carasa I, Cortes E, Honig LS, Dauer W, Consiglio A, Raya A, Sulzer D, Cuervo AM (2013) Interplay of LRRK2 with chaperone-mediated autophagy. Nat Neurosci 16(4):394–406. https://doi.org/10.1038/nn.3350
Pang SY, Ho PW, Liu HF, Leung CT, Li L, Chang EES, Ramsden DB, Ho SL (2019) The interplay of aging, genetics and environmental factors in the pathogenesis of Parkinson’s disease. Transl Neurodegener 8:23. https://doi.org/10.1186/s40035-019-0165-9
Patt S, Gertz HJ, Gerhard L, Cervos-Navarro J (1991) Pathological changes in dendrites of substantia nigra neurons in Parkinson’s disease: a Golgi study. Histol Histopathol 6(3):373–380
Pickhardt PC, Fisher NS (2007) Accumulation of inorganic and methylmercury by freshwater phytoplankton in two contrasting water bodies. Environ Sci Technol 41(1):125–131. https://doi.org/10.1021/es060966w
Reed MN, Newland MC (2009) Gestational methylmercury exposure selectively increases the sensitivity of operant behavior to cocaine. Behav Neurosci 123(2):408–417. https://doi.org/10.1037/a0014595
Saha S, Guillily MD, Ferree A, Lanceta J, Chan D, Ghosh J, Hsu CH, Segal L, Raghavan K, Matsumoto K, Hisamoto N, Kuwahara T, Iwatsubo T, Moore L, Goldstein L, Cookson M, Wolozin B (2009) LRRK2 modulates vulnerability to mitochondrial dysfunction in Caenorhabditis elegans. J Neurosci 29(29):9210–9218. https://doi.org/10.1523/jneurosci.2281-09.2009
Sawin ER, Ranganathan R, Horvitz HR (2000) C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26(3):619–631. https://doi.org/10.1016/s0896-6273(00)81199-x
Shores MM, White SS, Veith RC, Szot P (1999) Tyrosine hydroxylase mRNA is increased in old age and norepinephrine uptake transporter mRNA is decreased in middle age in locus coeruleus of Brown-Norway rats. Brain Res 826(1):143–147. https://doi.org/10.1016/s0006-8993(99)01200-7
Sierra M, Martínez-Rodríguez I, Sánchez-Juan P, González-Aramburu I, Jiménez-Alonso M, Sánchez-Rodríguez A, Berciano J, Banzo I, Infante J (2017) Prospective clinical and DaT-SPECT imaging in premotor LRRK2 G2019S-associated Parkinson disease. Neurology 89(5):439–444. https://doi.org/10.1212/wnl.0000000000004185
Strain JJ, Love TM, Yeates AJ, Weller D, Mulhern MS, McSorley EM, Thurston SW, Watson GE, Mruzek D, Broberg K, Rand MD, Henderson J, Shamlaye CF, Myers GJ, Davidson PW, van Wijngaarden E (2021) Associations of prenatal methylmercury exposure and maternal polyunsaturated fatty acid status with neurodevelopmental outcomes at 7 years of age: results from the Seychelles Child Development Study Nutrition Cohort 2. Am J Clin Nutr 113(2):304–313. https://doi.org/10.1093/ajcn/nqaa338
Tada Y, Marumoto K, Takeuchi A (2020) Nitrospina-like bacteria are potential mercury methylators in the mesopelagic zone in the East China Sea. Front Microbiol 11:1369. https://doi.org/10.3389/fmicb.2020.01369
Unoki T, Akiyama M, Kumagai Y, Gonçalves FM, Farina M, da Rocha JBT, Aschner M (2018) Molecular pathways associated with methylmercury-induced Nrf2 modulation. Front Genet 9:373. https://doi.org/10.3389/fgene.2018.00373
Vidal-Gadea A, Topper S, Young L, Crisp A, Kressin L, Elbel E, Maples T, Brauner M, Erbguth K, Axelrod A, Gottschalk A, Siegel D, Pierce-Shimomura JT (2011) Caenorhabditis elegans selects distinct crawling and swimming gaits via dopamine and serotonin. Proc Natl Acad Sci U S A 108(42):17504–17509. https://doi.org/10.1073/pnas.1108673108
Volta M, Beccano-Kelly DA, Paschall SA, Cataldi S, MacIsaac SE, Kuhlmann N, Kadgien CA, Tatarnikov I, Fox J, Khinda J, Mitchell E, Bergeron S, Melrose H, Farrer MJ, Milnerwood AJ (2017) Initial elevations in glutamate and dopamine neurotransmission decline with age, as does exploratory behavior, in LRRK2 G2019S knock-in mice. Elife. https://doi.org/10.7554/eLife.28377
Wagner GC, Reuhl KR, Ming X, Halladay AK (2007) Behavioral and neurochemical sensitization to amphetamine following early postnatal administration of methylmercury (MeHg). Neurotoxicology 28(1):59–66. https://doi.org/10.1016/j.neuro.2006.07.007
Weihe P, Grandjean P, Jørgensen PJ (2005) Application of hair-mercury analysis to determine the impact of a seafood advisory. Environ Res 97(2):200–207. https://doi.org/10.1016/j.envres.2004.01.006
Xiong Y, Neifert S, Karuppagounder SS, Liu Q, Stankowski JN, Lee BD, Ko HS, Lee Y, Grima JC, Mao X, Jiang H, Kang SU, Swing DA, Iacovitti L, Tessarollo L, Dawson TM, Dawson VL (2018) Robust kinase- and age-dependent dopaminergic and norepinephrine neurodegeneration in LRRK2 G2019S transgenic mice. Proc Natl Acad Sci U S A 115(7):1635–1640. https://doi.org/10.1073/pnas.1712648115
Xu X, Weber D, Martin A, Lone D (2016) Trans-generational transmission of neurobehavioral impairments produced by developmental methylmercury exposure in zebrafish (Danio rerio). Neurotoxicol Teratol 53:19–23. https://doi.org/10.1016/j.ntt.2015.11.003
Funding
This work was supported by the National Institute of Environmental Health Sciences (NIEHS) to MA and ABB (NIEHS R01ES007331 and R01ES10653). Some strains were provided by the CGC, which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440).
Author information
Authors and Affiliations
Contributions
Study conception and design: TK, ABB, and MA; data collection: TK; analysis and interpretation of results: TK, AAT, AS, MF, JBTR, ABB, and MA; draft manuscript preparation: TK, AAT, AVS, AS, MF, JBTR, ABB, and MA.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ke, T., Tinkov, A.A., Skalny, A.V. et al. The Human LRRK2 Modulates the Age-Dependent Effects of Developmental Methylmercury Exposure in Caenorhabditis elegans. Neurotox Res 40, 1235–1247 (2022). https://doi.org/10.1007/s12640-022-00547-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-022-00547-x