Abstract
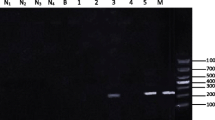
The present study aimed to evaluate a polymerase chain reaction (PCR) assay used for detection of Schistosoma mansoni infection in Biomphalaria alexandrina snails in early prepatent period and to compare between it and the ordinary detection methods (shedding and crushing). Biomphalaria alexandrina snails are best known for their role as intermediate hosts of S. mansoni. DNA was extracted from infected snails in addition to non-infected “negative control” (to optimized the efficiency of PCR reaction) and subjected to PCR using primers specific to a partial sequence of S. mansoni fructose-1,6-bus phosphate aldolase (SMALDO). SMALDO gene was detected in the infected laboratory snails with 70, 85, and 100 % positivity at the 1st, 3rd, and 7th day of infection, respectively. In contrast, the ordinary method was not sensitive enough in detection of early prepatent infection even after 7 days of infection which showed only 25 % positivity. By comparing the sensitivity of the three methods, it was found that the average sensitivity of shedding method compared to PCR was 23.8 % and the average sensitivity of crushing method compared to PCR was 46.4 % while the sensitivity of PCR was 100 %. We conclude that PCR is superior to the conventional methods and can detect positive cases that were negative when examined by shedding or crushing methods. This can help in detection of the areas and times of high transmission which in turn will be very beneficial in planning of the exact timing of the proper control strategy.



Similar content being viewed by others
References
Abath FG, Gomes Al, Melo Fl, Barbosa CS, Werkhauser RP (2006) Molecular approaches for the detection of Schistosoma mansoni: possible applications in the detection of snail infection, monitoring of transmission sites and diagnosis of human infection. Mem Inst Oswaldo Cruz Rio de Janeiro 101(Suppl. I):145–148
Abbasi I, King CH, Muchiri EM, Hamburger J (2010) Detection of Schistosoma mansoni and Schistosoma haematobium DNA by loop-mediated isothermal amplification: identification of infected snails from early prepatency. Am J Trop Med Hyg 83(2):427–432
Abu El Einin HM, Mansour WA, El-Dabaa E (2009) Assessment of infected Biomphalaria alexandrina snails by detecting Schistosoma mansoni antigen and specific gene. Aust J Basic Appl Sci 3(3):2747–2753
Behrman AJ (2005) Schistosomiasis. EMedicine. Available at: http://www.emedicine.com/emerg/topic857.htm. Accessed 31 March 2005
Caldeira RL, Jannotti-Passos LK, Lira PM, Carvalho OS (2004) Diagnostic of Biomphalaria Snails and Schistosoma mansoni: DNA obtained from traces of shell organic materials. Mem Inst Oswaldo Cruz Rio de Janeiro 99(5):499–502
Chu KY, Dawood v (1970) Cercarial production from Biomphalaria alexandrina infected with Schistosoma mansoni. Bull World Health Org 42:574–596
El-Dabaa E, Mei H, El-Sayed A, Karim AM, Eldesoky HM, Fahim FA, LoVerde PT, Saber MA (1998) Cloning and characterization of Schistosoma mansoni fructose-1,6-bisphosphate aldolase isozyme. J Parasitol 84:954–960
Fenwick A (2006) New initiatives against Africa’s worms. Trans R Soc Trop Med Hyg 100:200–207
Franco GR, Rabelo EML, Azevedo V, Pena HB, Ortega JM, Santos TM, Meira WSF, Rodrigues NA, Dias CMM, Harrop R, Wilson A, Saber MA, Abdel-Hamid H, Faria MSC, Margutti MEB, Parra JC, Pena SDJ (1997) Evaluation of cDNA libraries from different developmental stages of Schistosoma mansoni for production of expressed sequence tags (ESTs). DNA Res 4:231–240
Hahn UK, Bender RC, Bayne CJ (2001) Killing of Schistosoma mansoni sporocysts by hemocytes from resistant Biomphalaria glabrata: role of reactive oxygen species. J Parasitol 87:292–299
Hamburger J, Xin HX, Ramzy RM, Jourdane J, Ruppel A (1998) A polymerase chain reaction assay for detecting snails infected with Bilharzia parasites (Schistosoma mansoni) from very early prepatency. Am J Trop Med Hyg 59(6):872–876
Hamburger J, Hoffman O, Kariuki HC, Muchiri EM, Ouma JH, Koech DK, Sturrock RF, King CH (2004) Large scale polymerase chain reaction based surveillance of Schistosoma haematobium DNA in snails from transmission sites in Costal Kenya: a new tool for studying the dynamics of snail infection. Am J Trop Med Hyg 71(6):765–773
Hamed MA (2010) Strategic control of Schistosoma intermediate host. Asian J Epidemiol 3(3):123–140
Hanelt B, Adema CM, Mansour MH, Loker ES (1997) Detection of Schistosoma mansoni in Biomphalaria using nested PCR. J Paraitol 83:387–394
Humphries J (2010) Effects of Larval Schistosomes on Biomphalaria Snails. In: Toledo R, Fried B (eds) Biomphalaria snails and larval trematodes. Springer Verlag, New York, pp 103–125
Jannotti-Passos LK, Vidigal T, Dias-Neto E, Pena S, Simpson A, Dutra W, Souza C, Carvalho-Parra J (1997) PCR amplification of the mitochondrial DNA minisatellite region to detect Schistosoma mansoni infection in Biomphalaria glabrata snails. J Parasitol 83:395–399
Liang YS, John I, Bruce JI, David AB (1987) Laboratory cultivation of schistosome vector snails and maintenance of schistosome life cycle. Proc First Sine Am Symp 1:34
Melo FL, Gomes AV, Barbosa CS, Werkhauser RP, Abath FGC (2006) Development of molecular approaches for the identification of transmission sites of schistosomiasis. Trans R Soc Trop Med Hyg 100:1049–1055
Morgan JA, Dejong RJ, Snyder SD, Mkoji GM, Loker ES (2001) Schistosoma mansoni and Biomphalaria: past history and future trends. Parasitology 123(7):211–228
Negrao-Correa D, Mattos AC, Pereira CA, Martins-Souza RL, Coelho PM (2012) Interaction of Schistosoma mansoni sporocysts and hemocytes of Biomphalaria. J Parasitol Res. vol 2012
Pontes LA, Dias-Neto E, Rabello A (2002) Detection by polymerase chain reaction of Schistosoma mansoni DNA in human serum and feces. Am J Trop Med Hyg 66:157–162
Prah SK, James C (1977) The influence of physical factors on the behavior and infectivity of miracidia of S. mansoni and S. haematobium. II- effect of temperature and ultraviolet light. Helminthology 51:73–85
Sandoval N, Siles-Lucas M, Lopez AJ, Pérez-Arellano JL, Gárate T, Muro A (2006) Schistosoma mansoni: a diagnostic approach to detect acute schistosomiasis infection in a murine model by PCR. Exp Parasitol 114:84–88
Satayathum SA, Muchiri EM, Ouma JH, Whalen CC, King CH (2006) Factors affecting infection and reinfection with Schistosoma haematobium in coastal Kenya: survival analysis during nine-year, school-based treatment program. Am J Trop Med Hyg 75:83–92
Schmitt J, Wuhrer M, Hamburger J, Jourdane J, Ramzy RM, Gever R (2002) Schistosoma mansoni and Schistosoma haematobium: identification and characterization of glycoconjugate antigens in hemolymph of infected vector snails. J Parasitol 88:505–513
Webbe G (1965) Transmission of bilharziasis 2: production of cercariae. Bull World Health Orag 33:155–162
Webbe G, El Hak S (1990) Progress in the control of schistosomiasis in Egypt. Trans R Soc Trop Med Hyg 84:394–400
World Health Organization (2013) Schistosomiasis a major public health Available: http://www.who.int/schistosomiasis/en/(http://www-genome.wi.mit.edu/cgi-bin/primer/primer3_www.cgi)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farghaly, A., Saleh, A.A., Mahdy, S. et al. Molecular approach for detecting early prepatent Schistosoma mansoni infection in Biomphalaria alexandrina snail host. J Parasit Dis 40, 805–812 (2016). https://doi.org/10.1007/s12639-014-0583-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-014-0583-7