Abstract
Purpose
In children, the relationship between the dose of intraoperative opioid and postoperative outcomes is unclear. We examined the relationship between intraoperative opioid dose and postanesthesia care unit (PACU) pain scores and opioid and antiemetic administrations.
Methods
We performed a single-institution retrospective cohort study. Patients who were aged < 19 yr, had an American Society of Anesthesiologists Physical Status of I–III, were undergoing one of 11 procedures under general anesthesia and without regional anesthesia, and who were admitted to the PACU were included. Patients were analyzed by quartiles of total intraoperative opioid dose using multivariable regression, adjusting for confounders including procedure. An exploratory analysis of opioid-free anesthetics was also performed.
Results
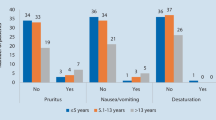
Three thousand, seven hundred and thirty-three cases were included, and the mean age of included patients was 8.3 yr. After adjustment, there were no significant differences between the lowest and higher quartiles for first conscious pain score, mean pain score, PACU opioid dose, or PACU length of stay; in addition, estimated differences were small. Patients in higher quartiles were estimated to be more likely to receive antiemetics, significantly so for those in the second quartile. Patients in the lowest quartile received significantly more intraoperative nonopioid analgesics. In the exploratory analysis, no significant difference in PACU pain scores was found in cases without intraoperative opioids.
Conclusions
Children who received lower doses of intraoperative opioids did not have worse PACU pain outcomes but required fewer antiemetics and received greater numbers of nonopioid analgesics intraoperatively. These findings suggest that lower doses of intraoperative opioids may be administered to children as long as other analgesics are used.
Résumé
Objectif
Chez les enfants, la relation entre la dose peropératoire d’opioïdes et les issues postopératoires n’est pas claire. Nous avons examiné la relation entre la dose peropératoire d’opioïdes, les scores de douleur en salle de réveil, et les administrations d’opioïdes et d’antiémétiques.
Méthode
Nous avons réalisé une étude de cohorte rétrospective dans un seul établissement. Nous avons inclus les patient·es âgé·es < 19 ans ayant un statut physique ASA de I-III et bénéficiant de l’une de 11 interventions sous anesthésie générale et sans anesthésie régionale, et qui avaient été admis·es en salle de réveil. Les patient·es ont été analysé·es par quartiles de la dose totale d’opioïdes peropératoires en utilisant une régression multivariée, en ajustant les données pour tenir compte des facteurs de confusion, notamment de l’intervention. Une analyse exploratoire des anesthésiques sans opioïdes a également été réalisée.
Résultats
Au total 3733 cas ont été inclus, et l’âge moyen des enfants était de 8,3 ans. Après ajustement, il n’y avait pas de différences significatives entre les quartiles inférieur et supérieur pour le premier score de douleur chez l’enfant conscient·e, le score de douleur moyen, la dose d’opioïdes en salle de réveil ou la durée du séjour en salle de réveil; de plus, les différences estimées étaient faibles. On a estimé que les patient·es des quartiles supérieurs étaient plus susceptibles de recevoir des antiémétiques et ce, de manière significative pour ceux et celles du deuxième quartile. Les patient·es du quartile inférieur ont reçu significativement plus d’analgésiques non opioïdes peropératoires. Dans l’analyse exploratoire, aucune différence significative dans les scores de douleur en salle de réveil n’a été trouvée dans les cas sans opioïdes peropératoires.
Conclusion
Les enfants qui ont reçu des doses plus faibles d’opioïdes peropératoires n’ont pas eu de pires issues de douleur en salle de réveil, mais ont eu besoin de moins d’antiémétiques et ont reçu un plus grand nombre d’analgésiques non opioïdes en peropératoire. Ces résultats suggèrent que des doses plus faibles d’opioïdes peropératoires peuvent être administrées aux enfants tant que d’autres analgésiques sont utilisés.



Similar content being viewed by others
References
Dahan A, Aarts L, Smith TW. Incidence, reversal, and prevention of opioid-induced respiratory depression. Anesthesiology 2010; 112: 226–38. https://doi.org/10.1097/aln.0b013e3181c38c25
de Boer HD, Detriche O, Forget P. Opioid-related side effects: postoperative ileus, urinary retention, nausea and vomiting, and shivering. A review of the literature. Best Pract Res Clin Anaesthesiol 2017; 31: 499–504. https://doi.org/10.1016/j.bpa.2017.07.002
Long DR, Lihn AL, Friedrich S, et al. Association between intraoperative opioid administration and 30-day readmission: a pre-specified analysis of registry data from a healthcare network in New England. Br J Anaesth 2018; 120: 1090–102. https://doi.org/10.1016/j.bja.2017.12.044
Zhu A, Benzon HA, Anderson TA. Evidence for the efficacy of systemic opioid-sparing analgesics in pediatric surgical populations: a systematic review. Anesth Analg 2017; 125: 1569–87. https://doi.org/10.1213/ane.0000000000002434
Rove KO, Edney JC, Brockel MA. Enhanced recovery after surgery in children: promising, evidence-based multidisciplinary care. Paediatr Anaesth 2018; 28: 482–92. https://doi.org/10.1111/pan.13380
Frauenknecht J, Kirkham KR, Jacot-Guillarmod A, Albrecht E. Analgesic impact of intra-operative opioids vs. opioid-free anaesthesia: a systematic review and meta-analysis. Anaesthesia 2019; 74: 651–62. https://doi.org/10.1111/anae.14582
Mann GE, Flamer SZ, Nair S, et al. Opioid-free anesthesia for adenotonsillectomy in children. Int J Pediatr Otorhinolaryngol 2021; 140: 110501. https://doi.org/10.1016/j.ijporl.2020.110501
Gilbertson LE, Patel C, De S, Lo W, Garcia-Roig M, Austin TM. The utilization of an opioid-free anesthetic for pediatric circumcision in an ambulatory surgery center. Children (Basel) 2021; 8: 678. https://doi.org/10.3390/children8080678
Kapila A, Glass PS, Jacobs JR, et al. Measured context-sensitive half-times of remifentanil and alfentanil. Anesthesiology 1995; 83: 968–75. https://doi.org/10.1097/00000542-199511000-00009
Nielsen S, Degenhardt L, Hoban B, Gisev N. A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiol Drug Saf 2016; 25: 733–7. https://doi.org/10.1002/pds.3945
Dowell D, Ragan KR, Jones CM, Baldwin GT, Chou R. CDC clinical practice guideline for prescribing opioids for pain—United States, 2022. MMWR Recomm Rep 2022; 71: 1–95. https://doi.org/10.15585/mmwr.rr7103a1
Wooldridge JM. Economic Analysis of Cross Section and Panel Data, 2nd ed. Cambridge: MIT Press; 2010
McCullagh P, Nelder JA. Generalized Linear Models, 2nd ed. London: Chapman and Hall; 1989
Gerbershagen HJ, Pogatzki-Zahn E, Aduckathil S, et al. Procedure-specific risk factor analysis for the development of severe postoperative pain. Anesthesiology 2014; 120: 1237–45. https://doi.org/10.1097/aln.0000000000000108
Yang MM, Hartley RL, Leung AA, et al. Preoperative predictors of poor acute postoperative pain control: a systematic review and meta-analysis. BMJ Open 2019; 9: e025091. https://doi.org/10.1136/bmjopen-2018-025091
Coppes OJ, Yong RJ, Kaye AD, Urman RD. Patient and surgery-related predictors of acute postoperative pain. Curr Pain Headache Rep 2020; 24: 12. https://doi.org/10.1007/s11916-020-0844-3
Pierre S, Whelan R. Nausea and vomiting after surgery. BJA Educ 2013; 13: 28–32. https://doi.org/10.1093/bjaceaccp/mks046
Lachenbruch PA. Power and sample size requirements for two-part models. Stat Med 2001; 20: 1235–8. https://doi.org/10.1002/sim.812
Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg 2017; 152: e170504. https://doi.org/10.1001/jamasurg.2017.0504
Nair AA, Velagapudi MA, Lang JA, et al. Machine learning approach to predict postoperative opioid requirements in ambulatory surgery patients. PLoS One 2020; 15: e0236833. https://doi.org/10.1371/journal.pone.0236833
Ward A, Jani T, De Souza E, Scheinker D, Bambos N, Anderson TA. Prediction of prolonged opioid use after surgery in adolescents: insights from machine learning. Anesth Analg 2021; 133: 304–13. https://doi.org/10.1213/ane.0000000000005527
Wanderer JP, Shi Y, Schildcrout JS, Ehrenfeld JM, Epstein RH. Supervising anesthesiologists cannot be effectively compared according to their patients’ postanesthesia care unit admission pain scores. Anesth Analg 2015; 120: 923–32. https://doi.org/10.1213/ane.0000000000000480
Author contributions
Michael R. King contributed to the conception and design of this study, analysis and interpretation of the data, and drafting the article. Elizabeth De Souza and Thomas A. Anderson contributed to the conception and design of this study, the acquisition, analysis and interpretation of data, and drafting the article.
Acknowledgements
The authors would like to thank Ellen Wang, MD, Clinical Associate Professor, Department of Anesthesiology, Perioperative and Pain Medicine, Lucile Packard Children's Hospital Stanford, Stanford University School of Medicine, Stanford, CA, USA for assistance with electronic medical record data management.
Disclosures
The authors have no conflicts of interest to disclose.
Funding
All research was performed using institutional funds alone.
Prior conference presentations
This work was presented at the 2022 Society for Pediatric Anesthesia and the American Academy of Pediatrics Spring Meeting (1–3 April, Tampa, Florida, USA) and the 2022 Association of University Anesthesiologists Annual Meeting (virtual).
Editorial responsibility
This submission was handled by Dr. Philippe Richebé, Associate Editor, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
King, M.R., De Souza, E. & Anderson, T.A. The association of intraoperative opioid dose with postanesthesia care unit outcomes in children: a retrospective study. Can J Anesth/J Can Anesth 71, 77–86 (2024). https://doi.org/10.1007/s12630-023-02612-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-023-02612-1