Abstract
Purpose
Cancer-related mortality, a leading cause of death worldwide, is often the result of metastatic disease recurrence. Anesthetic techniques have varying effects on innate and cellular immunity, activation of adrenergic-inflammatory pathways, and activation of cancer-promoting cellular signaling pathways; these effects may translate into an influence of anesthetic technique on long-term cancer outcomes. To further analyze the effects of propofol (intravenous) and volatile (inhalational gas) anesthesia on cancer recurrence and survival, we undertook a systematic review with meta-analysis.
Source
Databases were searched up to 14 November 2018. Comparative studies examining the effect of inhalational volatile anesthesia and propofol-based total intravenous anesthesia (TIVA) on cancer outcomes were included. The Newcastle Ottawa Scale (NOS) was used to assess methodological quality and bias. Reported hazard ratios (HRs) were pooled and 95% confidence intervals (CIs) calculated.
Principal findings
Ten studies were included; six studies examined the effect of anesthetic agent type on recurrence-free survival following breast, esophageal, and non-small cell lung cancer (n = 7,866). The use of TIVA was associated with improved recurrence-free survival in all cancer types (pooled HR, 0.78; 95% CI, 0.65 to 0.94; P < 0.01). Eight studies (n = 18,778) explored the effect of anesthetic agent type on overall survival, with TIVA use associated with improved overall survival (pooled HR, 0.76; 95% CI, 0.63 to 0.92; P < 0.01).
Conclusion
This meta-analysis suggests that propofol-TIVA use may be associated with improved recurrence-free survival and overall survival in patients having cancer surgery. This is especially evident where major cancer surgery was undertaken. Nevertheless, given the inherent limitations of studies included in this meta-analysis these findings necessitate prospective randomized trials to guide clinical practice.
Trial registration
PROSPERO (CRD42018081478); registered 8 October, 2018.
Résumé
Objectif
La mortalité liée au cancer, une cause majeure de décès dans le monde entier, est bien souvent le résultat de la récurrence de la maladie métastatique. Les techniques anesthésiques ont des effets variés sur l’immunité naturelle et cellulaire, l’activation des voies adrénergiques inflammatoires, et l’activation des voies de signalisation cellulaire promouvant le cancer; ces effets pourraient se traduire dans une influence de la technique anesthésique sur les pronostics de cancer à long terme. Afin d’approfondir l’analyse des effets de l’anesthésie au propofol (voie intraveineuse) et par inhalation (gaz) sur la récurrence du cancer et la survie, nous avons entrepris une revue systématique avec méta-analyse.
Source
Nous avons réalisé des recherches dans les bases de données jusqu’au 14 novembre 2018. Les études comparatives examinant l’effet d’une anesthésie par inhalation et d’une anesthésie intraveineuse totale (TIVA) avec propofol sur les pronostics de cancer ont été incluses dans notre revue. L’échelle de Newcastle-Ottawa (NOS) a été utilisée pour évaluer la qualité méthodologique et le biais. Les rapports de risque (RR) rapportés ont été pondérés et les intervalles de confiance (IC) à 95 % calculés.
Constatations principales
Dix études ont été incluses; six études ont examiné l’effet du type d’agent anesthésique sur la survie sans récurrence après un cancer du sein, de l’œsophage et du cancer pulmonaire non à petites cellules (n = 7866). L’utilisation d’une TIVA était associée à une amélioration de la survie sans récurrence, tous types de cancer confondus (RR pondéré, 0,78; IC 95 %, 0,65 à 0,94; P < 0,01). Huit études (n = 18 778) ont exploré l’effet du type d’agent anesthésique sur la survie globale, l’utilisation d’une TIVA étant alors associée à une amélioration de la survie globale (RR pondéré, 0,76; IC 95 %, 0,63 à 0,92; P < 0,01).
Conclusion
Cette méta-analyse suggère que l’administration d’une TIVA à base de propofol pourrait être associée à une amélioration de la survie sans récurrence et de la survie globale chez les patients subissant une chirurgie oncologique. Cette observation est particulièrement frappante dans les cas de chirurgie oncologique majeure. Toutefois, étant donné les lacunes inhérentes des études incluses dans cette méta-analyse, ces résultats nécessitent la réalisation d’études randomisées prospectives afin d’éclairer la pratique clinique.
Enregistrement de l’étude
PROSPERO (CRD42018081478); enregistrée le 8 octobre 2018.
Similar content being viewed by others
Cancer is one of the leading causes of death worldwide, with most patients dying from metastatic disease.1 It is currently estimated that more than 60% of patients with cancer will require surgery for the removal of solid tumours.2 The processes of tissue trauma, surgical manipulation of the tumour, and exposure to the physiologic stresses of the perioperative period can result in impaired local and cellular immunity, with consequent loco-regional recurrence and metastasis.3,4,5 The degree to which general anesthetic technique (inhalational volatile agents, or total intravenous anesthesia [TIVA] with propofol) contributes to this patient vulnerability in the perioperative period is an area of particular interest.5
Preclinical studies have found that both intravenous and volatile anesthetic agents alter the biology of cancer and immune cell lines by directly activating cellular receptors and cell signaling pathways, as well as by altering cellular kinetics and gene transcription.3,6,7 Anesthetic technique may also affect cell-mediated immunity and promote spread in different cancer types.8
A recent systematic review published by Soltanizadeh et al. examined outcomes of cancer surgery after inhalational vs intravenous anesthesia, but was constrained by the inclusion of studies that focused on postoperative complications or studies that did not have cancer recurrence or survival as the primary endpoint.9 We conducted this meta-analysis to provide an up-to-date assessment of the current evidence for the impact of type of anesthesia for cancer surgery on long-term clinical outcomes (cancer recurrence and overall survival).
Methods
Our results are reported using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA guidelines) and this review was registered on 8 October, 2018 with PROSPERO (registration number: CRD42018081478).
Study eligibility criteria
All randomized-controlled trials (RCTs) and observational longitudinal studies (prospective or retrospective) evaluating the effects of TIVA and inhalational anesthetic agents on cancer outcomes in patients undergoing cancer surgery were included. We excluded animal studies, studies not published in English, studies with insufficient information to perform the meta-analysis (such as no cancer-related endpoint), studies that did not report separate data for each intervention group with a measure of effect (such as hazard ratio [HR] or Peto odds ratio), or studies that focused on other interventions (e.g., perioperative chemotherapy and/or radiation therapy).
Information sources
The databases initially searched were Medline (through Ovid), EMBASE (through Ovid), The Cochrane Library, Web of Science, and PubMed from the start of inception until 17 March 2017, with a search update performed in PubMed and in sources of grey literature until 14 November 2018. Sources of grey literature included Open Grey and Google Scholar®. Conference proceedings and abstracts were searched in Web of Science. EndNote software (Clarivate Analytics, Philadelphia, PA, USA) was used to store all citations for duplicate checking.
Search
An experienced librarian (G.P.) developed a comprehensive search strategy that included broad terms such as “cancer”, “tumor”, “neoplasms”, “perioperative”, “anesthesia”, and narrow terms such as “TIVA”, “propofol”, “volatile”, and “sevoflurane” among other anesthetic intervention terms. The complete Medline search strategy and the terms for the search update are reported in Appendix 1.
Study selection
Eligibility assessments were performed independently by three teams of reviewers on behalf of the Global Onco-Anesthesia Research Collaboration Group (Acknowledgment). Cohen’s kappa coefficient, which describes the level of inter-rater agreement, was calculated. Disagreements at all stages were resolved through discussion. If agreement could not be reached, a third reviewer (B.R.) made a final decision.
Data collection process and data items
One reviewer (A.Y.) extracted data from individual studies and another reviewer (M.L.O.) cross-checked the information. The following information was extracted from each study: i) general information such as title, authors, publication year, and country; ii) study characteristics such as study design, setting, sample size, and outcome assessed; iii) participant characteristics such as number of patients within each group, age (mean and range) and type of cancer; and iv) intervention characteristics such as details of anesthetic techniques (volatile agents used, TIVA agent used), concomitant drug (opiates, anti-inflammatories and blood transfusions), or regional anesthesia use.
Risk of bias in individual studies
One reviewer (A.Y.) assessed the risk of bias of the included studies and another reviewer (B.R.) cross-checked the information. For RCTs, we used the Cochrane risk of bias tool. The potential of bias was appraised in five domains: selection, performance, detection, attrition, and reporting. These domains specifically evaluate how the random sequence was generated, methods of allocation concealment, blinding of participants and personnel, blinding of the outcome assessment, how incomplete outcome data were handled, and if there was evidence of selective outcome reporting. Each potential source of bias was graded as low, unclear, or high, and a justification for each judgment was provided. Observational studies were evaluated using the Newcastle Ottawa Scale (NOS),10 which assesses the potential for bias by scoring the selection process of the study groups, comparability of the groups, and ascertainment of exposure and outcome in the studies. Studies can be awarded a maximum of one point for each domain with an additional point being awarded for studies controlling for additional confounders. The maximum score allocated in the selection domain is 4 points, in the comparability domain is 2 points and outcome domain is 3 points. A maximum score of 9 points can be achieved and a higher score (≥ 7 points) indicates a lower risk of bias.11
Summary measures
The primary outcome measures for this meta-analysis were recurrence-free survival and overall survival. Adjusted HR from Cox proportional hazard models, their respective 95% confidence intervals (CI), and the P values were extracted from each of the studies. Where more than one data set was given, multivariate analysis data were used. If this was not provided, propensity matched data, if available, were used instead.
Synthesis of results
Analyses were conducted using Review Manager (RevMan version 5.3; Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). We converted the reported HRs into log HRs and used the generic inverse-variance method with random-effects model to pool the data. To maintain TIVA as a reference group across studies, we inverted the HR (1/HR) for studies reporting volatiles as a reference group. When data were provided in Kaplan Meier plots, an attempt was made to contact the study authors for further data. When the estimates differed substantially among the pooled studies (Chi squared test, P = 0.10), we conducted a sensitivity analysis by eliminating the outliers.
Additional analyses and risk of bias across studies
We a priori planned to explore sources of heterogeneity with subgroup analyses or meta-regression as well as using a funnel plot and a regression asymmetry test to assess small-study bias. Because of the small number of studies included, this was not done.
Results
Study selection
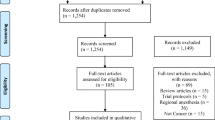
Figure 1 shows the study selection flowchart. We retrieved 12,508 citations and after removal of duplicates, there were 9,536 unique citations. After review, one prospective RCT and nine retrospective studies that pertained to volatile anesthesia and propofol-TIVA were included in the final analysis.12,13,14,15,16,17,18,19,20,21
Study characteristics and risk of bias within studies
Table 1 shows the study characteristics and Table 2 the recorded event rates (cancer recurrence or death). All the nine retrospective studies had a NOS ≥ 7 demonstrating good methodologic quality and a low risk of bias.12,13,14,15,16,17,18,20,21 The single prospective RCT included in this meta-analysis had a low risk of bias for each domain.19
Results of individual studies
Patients receiving a propofol infusion only, or a propofol and remifentanil infusion during their surgery were categorized into the TIVA group; patients receiving sevoflurane, isoflurane, desflurane or enflurane were categorized into the volatile group. All studies except Yan et al.19 adjusted for at least one of the following variables in their multivariate analyses: age, body mass index, comorbidities, preoperative therapy, pathologic stage or grade of cancer, and intraoperative anesthetic interventions such as epidural or blood transfusion.12,13,14,15,16,17,18,20,21
Synthesis of results
Recurrence-free survival
Six studies (five retrospective13,14,15,16,20 and one RCT)19 examined the effects of TIVA and volatile agents on recurrence-free survival in breast, esophageal, and non-small cell lung cancer (Fig. 2). The total sample size was 7,866 patients. When compared with volatile anesthesia, the use of TIVA was associated with improved recurrence-free survival in these cancer types (pooled HR, 0.78; 95% CI, 0.65 to 0.94; P < 0.01).
Overall survival
Eight studies (seven retrospective12,13,16,17,18,20,21 and one RCT),19 that included a total of 18,778 patients, provided ten HRs for this analysis (Fig. 3) in breast, colorectal, gastric, esophageal, and non-small cell lung cancer, and mixed cancer types. There was an associated improvement in overall survival with TIVA use when compared with volatile anesthesia (pooled HR, 0.76; 95% CI, 0.63 to 0.92; P < 0.01).
There was substantial heterogeneity among the studies and to explore it, outliers were removed (four estimates: Jun et al.,13 Wigmore et al.,17 Wu et al.18 and Zheng et al.).21 This gave an inconsistency score of 0% and a resulting pooled HR of 0.97 (95% CI, 0.85 to 1.11; P = 0.66). Unsuccessful strategies to further decrease the inconsistency score included removing the only RCT, removing the one study with multiple estimates (Enlund et al.),12 and leaving studies with positive estimates only.
Discussion
Despite advances in modern medicine, cancer is still a leading cause of death worldwide.22 It is therefore vital that clinicians consider all aspects of cancer care, including the delivery of anesthesia during cancer resection surgery, to optimize patients’ cancer outcomes. The pooled results from this meta-analysis suggest that TIVA use (compared with volatile anesthesia) during cancer surgery is associated with improved recurrence-free survival and overall survival across numerous cancer types. Breast cancer was the most often examined tumour type with five studies reporting on outcomes after breast cancer surgery12,14,15,19,20; in this population, TIVA use was associated with an improvement in recurrence-free survival but not overall survival.
The inconsistency in results between the individual studies included within the meta-analysis for breast cancer outcomes may be explained by confounding factors such as the degree of surgical trauma.23 In the study by Kim et al., where no benefit was reported with TIVA use across all types of breast cancer surgery, it is important to note that of those patients who had suffered recurrence, 73% of patients had undergone mastectomy.14 Similarly, in the study by Yoo et al., patients undergoing total mastectomy were associated with higher risks of cancer recurrence and all-cause mortality when compared with breast-conserving surgery.20 In the study by Lee et al., all of the patients underwent modified radical mastectomies and had a lower rate of cancer recurrence with TIVA use when compared with the sevoflurane group.15 Enlund et al. examined overall survival in patients with breast cancer but did not specify the type of surgical procedure.12 Importantly, the five-year survival rate for breast cancer is 88.9%, and thus the 50-70 month follow-up time in this study by Enlund et al.12 may not be sufficient to detect a meaningful difference of the effect of anesthesia type on survival.24
Preclinical studies suggest that drugs used for general anesthesia affect cellular immunity and potentiate cancer spread.3,25 Mechanistic studies have examined the differential effects of anesthetic agents on tumour cell biology, with in vitro data strongly supporting a pro-metastatic effect of volatile anesthesia and an anti-metastatic effect of propofol.3In vitro studies investigating the effect of different volatile agents have found an increased expression of cellular mediators that promote cancer cell proliferation, resistance of apoptosis by tumour cells, a propensity to invasion and migration of cells, endothelial-mesenchymal transition, basement membrane degradation, and angiogenesis.26,27,28,29,30,31 In contrast, when tumour cells are exposed to propofol, apoptosis is preserved and cell proliferation is reduced.32,33,34,35
Volatile anesthesia’s alteration of immune function has also been implicated in its hypothesized pro-metastatic potential through manipulation of the perioperative immune response.3 Preclinical data have reported impaired immune cell number and function after exposure to volatile anesthesia in animal models of cancer.3,25,36,37 Volatile agents reduce natural killer cell activity, a cytotoxic lymphocyte in the innate immune system and critical in the anti-tumour immune response.36,38,39 Reduced natural killer cell activity has been linked to tumour cell dissemination in patients with cancer.40,41,42 In contrast, in vitro studies report that propofol does not affect natural killer cell activity.39 Propofol may also reduce hypoxia-inducible factor 1α (HIF-1α) levels, a key regulator in the response to tumour growth.30 Activation of HIF-1α occurs during low oxygen states and promotes cell proliferation, angiogenesis, and metastasis43; this has been reported to be activated by volatile agents.26,30,44
It is therefore plausible that anesthesia technique is a critical component in cancer progression. Volatile agents may potentially “fuel the fire” and contribute to inherent cancer and surgical wounding processes characterized by pro-adrenergic, pro-inflammatory, immunomodulatory, and pro-angiogenic signalling.5 No single pathway, however, has been implicated, suggesting heterogeneity of the underlying drivers of cancer recurrence. Other clinically relevant interventions in the perioperative period, including surgical extent, blood transfusion, hypothermia, and administration of other medications (e.g., opioids, beta-blockers, anti-inflammatories, steroids), may themselves impact cancer cell biology.5
Surgical trauma activates neuroendocrine, inflammatory, immunologic, and metabolic pathways.45 These changes reduce innate and cellular immunity and may promote cancer spread postoperatively.46 Postoperative complications, including wound complications, pulmonary infections, and anastomotic leaks, have been reported to increase cancer recurrence and reduce overall survival.47,48 Such complications are characterized by exaggerated inflammatory processes. It is important to note that postoperative complications after cancer surgery have also been reported to be associated with anesthetic technique. De la Gala et al. noted a reduction in postoperative pulmonary complications and one year mortality with sevoflurane use (compared with, TIVA) in patients undergoing lung resection surgery.49 Conversely, Chang et al. noted fewer pulmonary complications and reduced mortality with TIVA use in patients with head and neck cancer undergoing free flap surgery when compared with volatile anesthesia.50 Postoperative complications may adversely affect postoperative recovery and reduce the ability to “Return to Intended Oncologic (adjuvant) Therapy” (RIOT) in the immediate postoperative period.51
Surveys of current clinical practice report that anesthesiologists generally have a preference for volatile anesthesia.52,53 In a survey of Australasian anesthesiologists, Lim et al. reported that > 80% of anesthesiologists prefer volatile-based anesthesia within their daily routine. Despite 43% of respondents reporting that they felt that TIVA may reduce cancer recurrence (compared with, inhalational anesthesia), only 29% reported regular use of TIVA for cancer surgery.52 This propensity toward volatile-based anesthesia necessitates large prospective RCTs of TIVA vs volatile anesthesia to inform international clinical guidelines.
Limitations of this study include the retrospective nature of the majority of the studies. The studies also had different follow-up intervals and significant variability of baseline patient demographics. There were also differences in study characteristics, including variable sample sizes in the treatment arms,13,14 unbalanced study populations (e.g., patients in one treatment group being older, having significant comorbidities),18 different stages/grades of cancer, differences in anesthetic technique (e.g., remifentanil, different volatiles used, and difference in use of regional anesthesia), and differences in surgical technique (e.g., differences in surgical magnitude). This study was also limited by the availability of data within the published manuscripts for analysis. The possibility of publication bias (which could not be assessed because the overall number of included studies was too low) should also be considered as this has the potential to greatly affect the results of this meta-analysis. Given these limitations, while the results favour a positive impact of propofol-based TIVA on cancer outcomes, the data should be interpreted with caution.
Collectively, this meta-analysis examined over 21,000 cancer patients with multiple cancer types. Despite the heterogeneity of the study designs and data, including different cancer types, there is an association between improved cancer outcomes with propofol-based TIVA when compared with inhalational volatile-based anesthesia. The results of this meta-analysis, together with the growing body of preclinical literature in the field, support the hypothesis that choice of anesthetic drug may influence patient outcome after cancer surgery. To test the hypothesis, a number of prospective RCTs in specific cancer types are currently underway (Randomized, Open-label Study to Compare Propofol Anesthesia With Sevoflurane Anesthesia in Terms of Overall Survival in Patients With Surgical Intervention for either Breast-, Colon-, or Rectal Cancer [NCT01975064]; General Anesthetics in CAncer REsection Surgery [GA-CARES] Trial: Pragmatic Randomized Trial of Propofol vs Volatile Inhalational Anesthesia [NCT03034096]; Impact of Inhalational Versus Intravenous Anesthesia Maintenance Methods on Long-term Survival Rate in Elderly Patients After Cancer Surgery: an Open-label, Randomized-Controlled Trial [NCT02660411]; and Volatile Anaesthesia and Perioperative Outcomes Related to Cancer [VAPOR-C]: A Feasibility Study [ACTRN1261700106538]) and will help guide the optimal anesthesia choice for perioperative cancer care.
Change history
22 May 2019
There was an isolated error relating to the Oh <Emphasis Type="Italic">et al.</Emphasis> study (1) within our recurrence-free survival analysis. When the reported estimates for Oh <Emphasis Type="Italic">et al.</Emphasis> are corrected, the pooled hazard ratio (HR) is now 0.87; 95% confidence interval (CI), 0.66 to 1.15; <Emphasis Type="Italic">P</Emphasis>=0.32.
References
Stewart BW, Wild C, International Agency for Research on Cancer; World Health Organization. World Cancer Report. Lyon, France - 2014. Geneva, Switzerland: International Agency for Research on Cancer WHO Press; 2014.
Sullivan R, Alatise OI, Anderson BO, et al. Global cancer surgery: delivering safe, affordable, and timely cancer surgery. Lancet Oncol 2015; 16: 1193-224.
Dubowitz JA, Sloan EK, Riedel BJ. Implicating anaesthesia and the perioperative period in cancer recurrence and metastasis. Clin Exp Metastasis 2018; 35: 347-58.
Dillekas H, Demicheli R, Ardoino I, Jensen SA, Biganzoli E, Straume O. The recurrence pattern following delayed breast reconstruction after mastectomy for breast cancer suggests a systemic effect of surgery on occult dormant micrometastases. Breast Cancer Res Treat 2016; 158: 169-78.
Hiller JG, Perry NJ, Poulogiannis G, Riedel B, Sloan EK. Perioperative events influence cancer recurrence risk after surgery. Nat Rev Clin Oncol 2018; 15: 205-18.
Ishikawa M, Tanaka S, Arai M, Genda Y, Sakamoto A. Differences in microRNA changes of healthy rat liver between sevoflurane and propofol anesthesia. Anesthesiology 2012; 117: 1245-52.
Sakamoto A, Imai J, Nishikawa A, et al. Influence of inhalation anesthesia assessed by comprehensive gene expression profiling. Gene 2005; 356: 39-48.
Byrne K, Levins KJ, Buggy DJ. Can anesthetic-analgesic technique during primary cancer surgery affect recurrence or metastasis? Can J Anesth 2016; 63: 184-92.
Soltanizadeh S, Degett TH, Gogenur I. Outcomes of cancer surgery after inhalational and intravenous anesthesia: a systematic review. J Clin Anesth 2017; 42: 19-25.
Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses - 2008. Available from URL: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed January 2019).
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25: 603-5.
Enlund M, Berglund A, Andreasson K, Cicek C, Enlund A, Bergkvist L. The choice of anaesthetic–sevoflurane or propofol–and outcome from cancer surgery: a retrospective analysis. Ups J Med Sci 2014; 119: 251-61.
Jun IJ, Jo JY, Kim JI, et al. Impact of anesthetic agents on overall and recurrence-free survival in patients undergoing esophageal cancer surgery: a retrospective observational study. Sci Rep 2017; 7: 14020.
Kim MH, Kim DW, Kim JH, Lee KY, Park S, Yoo YC. Does the type of anesthesia really affect the recurrence-free survival after breast cancer surgery? Oncotarget 2017; 8: 90477-87.
Lee JH, Kang SH, Kim Y, Kim HA, Kim BS. Effects of propofol-based total intravenous anesthesia on recurrence and overall survival in patients after modified radical mastectomy: a retrospective study. Korean J Anesthesiol 2016; 69: 126-32.
Oh TK, Kim K, Jheon S, et al. Long-term oncologic outcomes for patients undergoing volatile versus intravenous anesthesia for non-small cell lung cancer surgery: a retrospective propensity matching analysis. Cancer Control 2018; DOI: 10.1177/1073274818775360.
Wigmore TJ, Mohammed K, Jhanji S. Long-term survival for patients undergoing volatile versus IV anesthesia for cancer surgery: a retrospective analysis. Anesthesiology 2016; 124: 69-79.
Wu ZF, Lee MS, Wong CS, et al. Propofol-based total intravenous anesthesia is associated with better survival than desflurane anesthesia in colon cancer surgery. Anesthesiology 2018; 129: 932-41.
Yan T, Zhang GH, Wang BN, Sun L, Zheng H. Effects of propofol/remifentanil-based total intravenous anesthesia versus sevoflurane-based inhalational anesthesia on the release of VEGF-C and TGF-β and prognosis after breast cancer surgery: a prospective, randomized and controlled study. BMC Anesthesiol 2018; 18: 131.
Yoo S, Lee HB, Han W, et al. Total intravenous anesthesia versus inhalation anesthesia for breast cancer surgery: a retrospective cohort study. Anesthesiology 2019; 130: 31-40.
Zheng X, Wang Y, Dong L, et al. Effects of propofol-based total intravenous anesthesia on gastric cancer: a retrospective study. Onco Targets Ther 2018; 11: 1141-8.
World Health Organization. Cancer Fact Sheet. 2018 [cited 01/07/2018. Available from URL: http://www.who.int/news-room/fact-sheets/detail/cancer (accessed January 2019).
Sessler DI, Riedel B. Anesthesia and cancer recurrence: context for divergent study outcomes. Anesthesiology 2019; 130: 3-5.
National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Available from URL: https://seer.cancer.gov/ (accessed January 2019).
Yuki K, Bu W, Xi J, Shimaoka M, Eckenhoff R. Propofol shares the binding site with isoflurane and sevoflurane on leukocyte function-associated antigen-1. Anesth Analg 2013; 117: 803-11.
Iwasaki M, Zhao H, Jaffer T, et al. Volatile anaesthetics enhance the metastasis related cellular signalling including CXCR2 of ovarian cancer cells. Oncotarget 2016; 7: 26042-56.
Wu GJ, Chen WF, Sung CS, et al. Isoflurane attenuates dynorphin-induced cytotoxicity and downregulation of Bcl-2 expression in differentiated neuroblastoma SH-SY5Y cells. Acta Anaesthesiol Scand 2009; 53: 55-60.
Ecimovic P, McHugh B, Murray D, Doran P, Buggy DJ. Effects of sevoflurane on breast cancer cell function in vitro. Anticancer Res 2013; 33: 4255-60.
Kawaraguchi Y, Horikawa YT, Murphy AN, et al. Volatile anesthetics protect cancer cells against tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis via caveolins. Anesthesiology 2011; 115: 499-508.
Huang H, Benzonana LL, Zhao H, et al. Prostate cancer cell malignancy via modulation of HIF-1alpha pathway with isoflurane and propofol alone and in combination. Br J Cancer 2014; 111: 1338-49.
Deegan CA, Murray D, Doran P, Ecimovic P, Moriarty DC, Buggy DJ. Effect of anaesthetic technique on oestrogen receptor-negative breast cancer cell function in vitro. Br J Anaesth 2009; 103: 685-90.
Zhang D, Zhou XH, Zhang J, et al. Propofol promotes cell apoptosis via inhibiting HOTAIR mediated mTOR pathway in cervical cancer. Biochem Biophys Res Commun 2015; 468: 561-7.
Wu KC, Yang ST, Hsu SC, et al. Propofol induces DNA damage in mouse leukemic monocyte macrophage RAW264.7 cells. Oncol Rep 2013; 30: 2304-10.
Ye Z, Jingzhong L, Yangbo L, Lei C, Jiandong Y. Propofol inhibits proliferation and invasion of osteosarcoma cells by regulation of microRNA-143 expression. Oncol Res 2013; 21: 201-7.
Wu KC, Yang ST, Hsia TC, et al. Suppression of cell invasion and migration by propofol are involved in down-regulating matrix metalloproteinase-2 and p38 MAPK signaling in A549 human lung adenocarcinoma epithelial cells. Anticancer Res 2012; 32: 4833-42.
Stollings LM, Jia LJ, Tang P, Dou H, Lu B, Xu Y. Immune modulation by volatile anesthetics. Anesthesiology 2016; 125: 399-411.
Yuki K, Eckenhoff RG. Mechanisms of the immunological effects of volatile anesthetics: a review. Anesth Analg 2016; 123: 326-35.
Buckley A, McQuaid S, Johnson P, Buggy DJ. Effect of anaesthetic technique on the natural killer cell anti-tumour activity of serum from women undergoing breast cancer surgery: a pilot study. Br J Anaesth 2014; 113(Suppl 1): i56-62.
Melamed R, Bar-Yosef S, Shakhar G, Shakhar K, Ben-Eliyahu S. Suppression of natural killer cell activity and promotion of tumor metastasis by ketamine, thiopental, and halothane, but not by propofol: mediating mechanisms and prophylactic measures. Anesth Analg 2003; 97: 1331-9.
Takeuchi H, Maehara Y, Tokunaga E, Koga T, Kakeji Y, Sugimachi K. Prognostic significance of natural killer cell activity in patients with gastric carcinoma: a multivariate analysis. Am J Gastroenterol 2001; 96: 574-8.
Ben-Eliyahu S, Page GG, Yirmiya R, Shakhar G. Evidence that stress and surgical interventions promote tumor development by suppressing natural killer cell activity. Int J Cancer 1999; 80: 880-8.
Seth R, Tai LH, Falls T, et al. Surgical stress promotes the development of cancer metastases by a coagulation-dependent mechanism involving natural killer cells in a murine model. Ann Surg 2013; 258: 158-68.
Ziello JE, Jovin IS, Huang Y. Hypoxia-inducible factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. Yale J Biol Med 2007; 80: 51-60.
Benzonana LL, Perry NJ, Watts HR, et al. Isoflurane, a commonly used volatile anesthetic, enhances renal cancer growth and malignant potential via the hypoxia-inducible factor cellular signaling pathway in vitro. Anesthesiology 2013; 119: 593-605.
Kehlet H. Manipulation of the metabolic response in clinical practice. World J Surg 2000; 24: 690-5.
Lennard TW, Shenton BK, Borzotta A, et al. The influence of surgical operations on components of the human immune system. Br J Surg 1985; 72: 771-6.
Beecher SM, O’Leary DP, McLaughlin R, Sweeney KJ, Kerin MJ. Influence of complications following immediate breast reconstruction on breast cancer recurrence rates. Br J Surg 2016; 103: 391-8.
Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg 2011; 253: 890-9.
de la Gala F, Pineiro P, Reyes A, et al. Postoperative pulmonary complications, pulmonary and systemic inflammatory responses after lung resection surgery with prolonged one-lung ventilation. Randomized controlled trial comparing intravenous and inhalational anaesthesia. Br J Anaesth 2017; 119: 655-63.
Chang YT, Wu CC, Tang TY, Lu CT, Lai CS, Shen CH. Differences between total intravenous anesthesia and inhalation anesthesia in free flap surgery of head and neck cancer. PLoS One 2016; 11: e0147713.
Aloia TA, Zimmitti G, Conrad C, Gottumukalla V, Kopetz S, Vauthey JN. Return to intended oncologic treatment (RIOT): a novel metric for evaluating the quality of oncosurgical therapy for malignancy. J Surg Oncol 2014; 110: 107-14.
Lim A, Braat S, Hiller J, Riedel B. Inhalational versus propofol-based total intravenous anaesthesia: practice patterns and perspectives among Australasian anaesthetists. Anaesth Intensive Care 2018; 46: 480-7.
Pandit JJ, Andrade J, Bogod DG, et al. 5th National Audit Project (NAP5) on accidental awareness during general anaesthesia: summary of main findings and risk factors. Br J Anaesth 2014; 113: 549-59.
Acknowledgements
This work is funded by the Australian and New Zealand College of Anaesthetists Foundation (18/038). Dr. Lopez-Olivo’s work is supported by the Rheumatology Research Foundation. Dr. Dubowitz’s work is funded by Monash University PhD and ANZCA scholarships. The authors would like to thank Greg Pratt (The University of Texas M.D. Anderson Cancer Center, Houston, Texas, USA) for support with the literature searches.
Global Onco-Anesthesia Research Collaboration Group: Group 1 Bernhard Riedel—Peter MacCallum Cancer Centre, University of Melbourne and Monash University, Australia, Timothy Wigmore—The Royal Marsden NHS Trust, London, UK, Julia Dubowitz—Peter MacCallum Cancer Centre and Monash University, Australia, Marissa Ferguson—Peter MacCallum Cancer Centre, Melbourne, Australia, David Shan—Peter MacCallum Cancer Centre, Melbourne, Australia, Ken Yee—Peter MacCallum Cancer Centre, Melbourne, Australia. Group 2 Jonathan Hiller—Peter MacCallum Cancer Centre, University of Melbourne and Monash University, Australia, Ilonka Meyer—Peter MacCallum Cancer Centre, Melbourne, Australia, Andrea Yap—National University Hospital, Singapore, Peter MacCallum Cancer Centre, Melbourne, Australia. Group 3 Robert Schier—Department of Anaesthesiology and Intensive Care Medicine, University Hospital of Cologne, Cologne, Germany, Vijaya Gottumukkala—The University of Texas MD Anderson Cancer Centre, Houston, USA, Jonathan Wilks—The Royal Marsden NHS Trust, London, UK, Volker Schick—Department of Anaesthesiology and Intensive Care Medicine, University Hospital of Cologne, Cologne, Germany, Victor Hui—Peter MacCallum Cancer Centre, Melbourne, Australia. Other members Erica Sloan—Drug Discovery Biology Theme, Monash Institute of Pharmaceutical Sciences, Monash University, Melbourne, London and Cousins Center for PNI, Semel Institute for Neuroscience and Human Behavior, University of California Los Angeles, USA, Juan Cata—The University of Texas MD Anderson Cancer Centre, Houston, USA, Donal Buggy—Department of Anaesthesia, Mater University Hospital, University College Dublin, Ireland.
Author contributions
Andrea Yap contributed to all aspects of this manuscript, including conception and design; acquisition, analysis, and interpretation of data and drafting the article. Maria A. Lopez-Olivo contributed to acquisition, analysis, interpretation of data and drafting the article. Julia Dubowitz and Jonathan Hiller contributed to analysis, interpretation of data and drafting the article. Bernhard Riedel contributed to conception and design, analysis, and interpretation of data and drafting the article.
Competing interests
None declared.
Editorial responsibility
This submission was handled by Dr. Hilary P. Grocott, Editor-in-Chief, Canadian Journal of Anesthesia.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is accompanied by an editorial. Please see Can J Anesth 2019; 66: this issue.
Appendix 1 Search strategies
Appendix 1 Search strategies
Medline (Ovid) – Inception until March 17, 2017
1 | exp NEOPLASMS/su |
2 | ((cancer* or neoplas* or malignan* or tumor* or tumour* or metasta* or carcinoma* or oncolog* or recurrence* or chemotherapy* or chemo-therap* or antineoplas* or anti-neoplas*) and (patient* or surg* or resect*)).ti. |
3 | ((cancer* or neoplas* or malignan* or tumor* or tumour* or metasta* or carcinoma* or oncolog* or recurrence* or chemotherapy* or chemo-therap* or antineoplas* or anti-neoplas*) adj5 (patient* or surg* or resect*)).ti,ab. |
4 | or/1-3 |
5 | exp PERIOPERATIVE PERIOD/ |
6 | exp PERIOPERATIVE CARE/ |
7 | (perioperativ* or peri-operativ* or intraoperativ* or intra-operativ*).ti. |
8 | ((pre-surg* or presurg* or post-surg* or postsurg* or preoperativ* or pre-operativ* or postoperativ* or post-operativ*) and (anesthe* or anaesthe* or analges* or block* or post-anesthe* or postanesthe* or post-anaesthe* or postanaesthe* or pre-anesthe* or preanesthe* or pre-anaesthe* or preanaesthe* or “fast track”)).ti. |
9 | (((perioperativ* or peri-operativ* or intraoperativ* or intra-operativ* or pre-surg* or presurg* or post-surg* or postsurg* or preoperativ* or pre-operativ* or postoperativ* or post-operativ* or “fast track”) adj5 (anesthe* or anaesthe* or analges* or block* or post-anesthe* or postanesthe* or post-anaesthe* or postanaesthe* or pre-anesthe* or preanesthe* or pre-anaesthe* or preanaesthe*)) and (cancer* or neoplas* or malignan* or tumor* or tumour* or metasta* or carcinoma* or oncolog* or recurrence* or chemotherapy* or chemo-therap* or antineoplas* or anti-neoplas*)).ab. |
10 | or/5-9 |
11 | 4 and 10 [Ca surg/pts + periop] |
12 | exp *PERIOPERATIVE PERIOD/ or exp *PERIOPERATIVE CARE/ or (perioperativ* or peri-operativ* or intraoperativ* or intra-operativ* or pre-surg* or presurg* or post-surg* or postsurg* or preoperativ* or pre-operativ* or postoperativ* or post-operativ* or post-anesthe* or postanesthe* or post-anaesthe* or postanaesthe* or pre-anesthe* or preanesthe* or pre-anaesthe* or preanaesthe*).ti. [periop focus] |
13 | exp *NEOPLASMS/su or (cancer* or neoplas* or malignan* or tumor* or tumour* or metasta* or carcinoma* or oncolog* or recurrence* or chemotherapy* or chemo-therap* or antineoplas* or anti-neoplas*).ti. [Ca focus] |
14 | exp *”ANESTHESIA AND ANALGESIA”/ or (anesthe* or anaesthe* or analges* or TIVA or ((nerve* or regional* or spinal* or neuraxial* or paravertebral*) adj3 block*)).ti. or (total* adj intravenous* adj3 (anesthe* or anaesthe* or technique*)).ti. [Anes focus] |
15 | ((perioperativ* or peri-operativ* or intraoperativ* or intra-operativ* or pre-surg* or presurg* or post-surg* or postsurg* or preoperativ* or pre-operativ* or postoperativ* or post-operativ* or post-anesthe* or postanesthe* or post-anaesthe* or postanaesthe* or pre-anesthe* or preanesthe* or pre-anaesthe* or preanaesthe*) adj5 (cancer* or neoplas* or malignan* or tumor* or tumour* or metasta* or carcinoma* or oncolog* or recurrence* or chemotherapy* or chemo-therap* or antineoplas* or anti-neoplas* or ((nerve* or regional* or spinal* or neuraxial* or paravertebral*) adj3 block*) or (total* adj intravenous* adj3 (anesthe* or anaesthe* or technique*)))).ab. |
16 | (12 and 13) or (13 and 14) or (12 and 14) [#1 focus] |
17 | (12 and 13) or (13 and 14) or (12 and 14) or (15 and (12 or 13 or 14)) [#2 focus] |
18 | exp “ANESTHESIA AND ANALGESIA”/ |
19 | (anesthe* or anaesthe* or analges* or TIVA or (total* adj intravenous* adj3 (anesthe* or anaesthe* or technique*))).ti. |
20 | ((nerve* or regional* or spinal* or neuraxial* or paravertebral*) adj3 block*).ti. |
21 | ((anesthe* or anaesthe* or analges* or block*) adj3 (technique* or regional* or epidural* or peridural* or spinal* or neuraxial* or paravertebral*)).ab. |
22 | ((nerve* or regional* or spinal* or neuraxial* or paravertebral*) adj3 block*).ab. |
23 | ((analges* adj3 patient* adj3 control*) or TIVA or (total* adj intravenous* adj3 (anesthe* or anaesthe* or technique*))).ab. |
24 | or/18-23 [anes or analg or block terms] |
25 | 11 and 24 [Ca surg/pts + periop + (anes or analg or block)] |
26 | exp ANESTHETICS/ or exp ANALGESICS, OPIOID/ or MORPHINE/ or HALOTHANE/ or ISOFLURANE/ or KETAMINE/ or THIOPENTAL/ or PROPOFOL/ |
27 | ((volatile* or inhal* or induc*) and (anesthe* or anaesthe* or analges*)).ti. |
28 | ((volatile* or inhal* or induc*) adj3 (anesthe* or anaesthe* or analges*)).ab. |
29 | (lignocaine* or lidocaine* or bupivacaine* or ropivicaine* or clonidine* or morphine* or pethidine* or naloxone* or nalbuphine* or naltrexone* or fentanyl* or alfentanil* or sufentanil* or remifentanil* or codeine* or hydrocodone* or oxycodone* or demerol* or tramadol*).ti,rn. |
30 | (halothane* or enflurane* or flurane* or isofulrane* or sevoflurane* or desflurane* or ketamine* or thiopentone* or thiopental* or etomidate* or propofol* or (nitrous adj oxide*)).ti,rn. |
31 | or/26-30 [anesthetic MeSH or KW terms] |
32 | 11 and 31 [Ca surg/pts + periop + anesthetics] |
33 | exp ADRENERGIC BETA-ANTAGONISTS/ or PROPRANOLOL/ or ISOPROTERENOL/ or ATENOLOL/ or BISOPROLOL/ or METOPROLOL/ or exp ADRENERGIC ALPHA-AGONISTS/ or CLONIDINE/ or DEXMEDETOMIDINE/ |
34 | ((beta adj5 (blocker* or blocking)) or (adrenergic* adj5 beta adj5 (block* or antagonist*)) or (adrenergic* adj5 alpha adj5 agonist*)).ti. |
35 | (propanolol* or Isoproterenol* or atenolol* or bisoprolol* or metoprolol* or clonidine* or dexmedetomidine*).ti,rn. |
36 | ((beta adj3 (blocker* or blocking)) or (adrenergic* adj3 beta adj3 (block* or antagonist*)) or (adrenergic* adj3 alpha adj3 agonist*)).ab. |
37 | or/33-36 [beta-blocker terms] |
38 | 11 and 37 [Ca + periop + beta-blockers] |
39 | exp *ANTI-INFLAMMATORY AGENTS, NON-STEROIDAL/ or Anti-Inflammatory Agents, Non-Steroidal.rn. or *KETOROLAC/ or *DICLOFENAC/ or *ACETAMINOPHEN/ or *IBUPROFEN/ or *ASPIRIN/ or exp *SERINE PROTEINASE INHIBITORS/ or *APROTININ/ [NSAID etc MeSH MJ] |
40 | (((nonsteroidal or non-steroidal) adj3 anti-inflammatory adj3 (drug* or agent*)) or ((COX or COX2 or cyclooxygenase* or cyclo-oxygenase*) adj5 inhibit*) or (serine* adj5 (protease* or proteinase*) adj5 inhibit*)).ti. |
41 | (NSAID* or aspirin* or ketorolac* or diclofenac* or etedolac* or acetaminophen* or tylenol* or paracetamol* or ibuprofen* or celecoxib* or parecoxib* or rofecoxib* or valdecoxib* or etoricoxib* or gabapentin* or pregabalin* or aprotinin*).ti,rn. |
42 | (((COX or COX2 or cyclooxygenase* or cyclo-oxygenase*) adj3 inhibit*) or (serine* adj3 (protease* or proteinase*) adj3 inhibit*)).ab. |
43 | (LIDOCAINE/ or MAGNESIUM/) and (ANESTHESIA, INTRAVENOUS/ or exp ADMINISTRATION, INTRAVENOUS/) |
44 | ((lidocaine* or xylocaine* or magnesium*) adj5 (intravenous* or IV*1)).ti. or ((lidocaine* or xylocaine* or magnesium*) adj3 (intravenous* or IV*1)).ab. or ((nonsteroidal or non-steroidal) adj3 anti-inflammatory adj3 (drug* or agent*)).ab. |
45 | or/39-44 [NSAIDs and locals] |
46 | 11 and 45 [Ca + periop + (NSAIDs or locals)] |
47 | 25 or 32 or 38 or 46 |
48 | exp *BLOOD TRANSFUSION/ or BLOOD LOSS, SURGICAL/ or exp POSTOPERATIVE HEMORRHAGE/ |
49 | ((transfus* and (blood* or erythrocyt* or leukocyt* or platelet* or plasma*)) or (blood and (loss or lost or losing or product*)) or (hemorrag* or haemorrag* or bleed* or hemosta* or hemodynamic* or haemosta* or haemodynamic*)).ti. |
50 | ((perioperativ* or peri-operativ* or intraoperativ* or intra-operativ* or pre-surg* or presurg* or post-surg* or postsurg* or preoperativ* or pre-operativ* or postoperativ* or post-operativ* or post-anesthe* or postanesthe* or post-anaesthe* or postanaesthe* or pre-anesthe* or preanesthe* or pre-anaesthe* or preanaesthe*) adj3 (transfus* or autotransfus* or auto-transfus* or hemorrag* or haemorrag* or bleed* or (blood adj3 (loss or lost or losing or product*)))).ab. |
51 | 16 and 50 [adding #1 focus] |
52 | 48 or 49 or 51 [transfusion/blood loss terms] |
53 | 11 and 52 [Ca + periop + (transfusion or blood loss)] |
54 | BLOOD GLUCOSE/ or exp HYPERGLYCEMIA/ or exp HYPOGLYCEMIC AGENTS/ or GLUCOSE TOLERANCE TEST/ or HEMOGLOBIN A, GLYCOSYLATED/ or exp DIABETES MELLITUS/ or exp HYPERINSULINISM/ or exp INSULIN/ |
55 | ((blood* adj3 glucose*) or euglycemi* or euglycaemi* or HbA1c or “Hb A1c” or “hemoglobin A1c” or diabet* or NIDDM or IDDM or T2DM).ti. |
56 | ((control* or manag* or treat* or monitor* or regulat* or regimen* or protocol*) and (glucose* or glycemi* or glycaemi* or insulin*1 or hyperglycemi* or hyperglycaemi*)).ti. |
57 | (((perioperativ* or peri-operativ* or intraoperativ* or intra-operativ* or pre-surg* or presurg* or post-surg* or postsurg* or preoperativ* or pre-operativ* or postoperativ* or post-operativ* or intraoperativ* or intra-operativ* or post-anesthe* or postanesthe* or post-anaesthe* or postanaesthe* or pre-anesthe* or preanesthe* or pre-anaesthe* or preanaesthe*) adj10 ((blood* adj3 glucose*) or euglycemi* or euglycaemi* or HbA1c or “Hb A1c” or “hemoglobin A1c” or diabet* or NIDDM or IDDM or T2DM)) or ((control* or manag* or treat* or monitor* or regulat* or regimen* or protocol*) adj5 (glucose* or glycemi* or glycaemi* or insulin*1 or hyperglycemi* or hyperglycaemi*))).ab. |
58 | or/54-57 [blood glucose terms] |
59 | 11 and 58 [Ca + periop + blood glucose] |
60 | exp BODY TEMPERATURE/ or exp BODY TEMPERATURE CHANGES/ or exp HYPOTHERMIA, INDUCED/ or MALIGNANT HYPERTHERMIA/ or REWARMING/ |
61 | ((body* adj3 temperature*) or sweat* or shiver* or fever* or hyperthermi* or hypothermi* or normothermi* or rewarm*).ti. |
62 | (((malignan* or anesthes* or anaesthes*) adj5 (hyperthermi* or hyperpyrexi*)) or (induc* adj5 hypothermi*)).ti. |
63 | ((body* adj3 temperature*) or sweat* or shiver* or fever* or hyperthermi* or hypothermi* or normothermi* or rewarm* or ((malignan* or anesthes* or anaesthes*) adj5 (hyperthermi* or hyperpyrexi*)) or (induc* adj5 hypothermi*)).ab. |
64 | or/60-62 |
65 | 63 and (12 or 14) [ab kw + periop or anes focus] |
66 | 64 or 65 [body temp terms] |
67 | 11 and 66 [Ca + periop + body temp] |
68 | exp STEROIDS/ or GLUCOCORTICOIDS/ |
69 | (corticosteroid* or glucocorticoid* or glucocorticosteroid* or steroid*).ti,rn. |
70 | (cortisone* or dexamethasone* or hydrocortisone* or methylprednisolone* or prednisolone* or prednisone*).ti,rn. |
71 | or/68-70 [steroid terms] |
72 | 11 and 71 [Ca + periop + steroids] |
73 | (anesth* or analg* or anaesth* or pain*).in,jw. [anes term in author address or journal name] |
74 | 11 and 73 [Ca + Periop + anes term in au addrs or jrnl name] |
75 | 25 or 32 or 38 or 46 or 53 or 59 or 67 or 72 or 74 |
76 | (animals not (humans and animals)).sh. |
77 | 75 not 76 [removing “animal-only” studies] |
78 | (case reports not (case reports and review)).pt. |
79 | case report*.ti. not review.pt. |
80 | 78 or 79 [case reports not part of a review] |
81 | 77 not 80 [removing case reports unless part of a review] |
82 | exp CLINICAL TRIAL/ or exp CLINICAL TRIALS AS TOPIC/ or DRUG EVALUATION/ or exp EPIDEMIOLOGIC RESEARCH DESIGN/ or exp EPIDEMIOLOGIC STUDIES/ or COMPARATIVE EFFECTIVENESS RESEARCH/ [MeSH study terms] |
83 | 81 and 82 [most likely CTs] |
84 | (randomized-controlled trial or rct* or multicenter study or controlled clinical trial or clinical trial or ((single or double or triple or treble) adj3 (blind* or dummy or mask*)) or ((random* or control* or clinical*) adj3 (study or studies or studied or trial*)) or ((multicent* or retrospective* or prospective*) adj3 (study or studies or studied or trial* or design*))).ti,ab. |
85 | 81 and 84 [contain a CT term] |
86 | observational study.pt. or ((observ* or longitudinal* or open-label or cross-over or crossover or cross-section* or cohort* or comparative or comparison) adj3 (study or studies or studied or trial* or design*)).ti,ab. |
87 | 81 and 86 |
88 | comparative study.pt. or (case*1 adj3 series).ti,ab. or (drug* adj3 (compar* or evaluat*)).ti,ab. or “head-to-head”.ti,ab. or (meta-analy* or met-analy*).ti,ab. or (meta-regression* or mega-regession*).ti,ab. or ((systematic* adj3 review*) or “systematic overview*”).ti,ab. or ((methodologic* adj3 review*) or “methodologic* overview*”).ti,ab. or (quantitative* adj3 (review* or synthes*)).ti,ab. or (research adj3 (integrat* or overview*)).ti,ab. or ((integrative* or collaborative*) adj3 (overview* or review*)).ti,ab. or (pool* adj3 analys*).ti,ab. or (data adj3 (synthes* or extract* or abstract*)).ti,ab. |
89 | 81 and 88 |
90 | 81 and META-ANALYSIS/ |
91 | limit 81 to systematic reviews [SR as publication type] |
92 | 83 or 85 or 87 or 89 or 90 or 91 |
Medline (PubMed) – 2017 to November 13, 2018
1 | Search “NEOPLASMS/surgery”[Mesh] |
2 | Search (cancer*[tiab] or neoplas*[tiab] or malignan*[tiab] or tumor*[tiab] or tumour*[tiab] or metasta*[tiab] or carcinoma*[tiab] or oncolog*[tiab] or recurrence*[tiab] or chemotherapy*[tiab] or chemo-therap*[tiab] or antineoplas*[tiab] or anti-neoplas*[tiab]) and (patient[tiab] or patients[tiab] or surg*[tiab] or resect*[tiab]) |
3 | Search (cancer*[tiab] or neoplas*[tiab] or malignan*[tiab] or tumor*[tiab] or tumour*[tiab] or metasta*[tiab] or carcinoma*[tiab] or oncolog*[tiab] or recurrence*[tiab] or chemotherapy*[tiab] or chemo-therap*[tiab] or antineoplas*[tiab] or anti-neoplas*[tiab]) AND (patient[tiab] or patients[tiab] or surg*[tiab] or resect*[tiab]) |
4 | Search #1 OR #2 OR #3 |
5 | Search “PERIOPERATIVE PERIOD”[Mesh] |
6 | Search “PERIOPERATIVE CARE”[Mesh] |
7 | Search perioperativ*[tiab] or peri-operativ*[tiab] or intraoperativ*[tiab] or intra-operativ*[tiab] |
8 | Search ((pre-surg*[tiab] or presurg*[tiab] or post-surg*[tiab] or postsurg*[tiab] or preoperativ*[tiab] or pre-operativ*[tiab] or postoperativ*[tiab] or post-operativ*[tiab]) and (anesthe*[tiab] or anaesthe*[tiab] or analges*[tiab] or block*[tiab] or post-anesthe*[tiab] or postanesthe*[tiab] or post-anaesthe*[tiab] or postanaesthe*[tiab] or pre-anesthe*[tiab] or preanesthe*[tiab] or pre-anaesthe*[tiab] or preanaesthe*[tiab] or “fast track”[tiab])) |
9 | Search #5 OR #6 OR #7 OR #8 |
10 | Search #4 AND #9 |
11 | Search “ANESTHESIA, INTRAVENOUS”[Mesh] and total[tiab] |
12 | Search “total intravenous anesthesia”[tiab] or “total intravenous anaesthesia”[tiab] or “total intravenous technique”[tiab] |
13 | Search tiva[tiab] |
14 | Search “HALOTHANE”[Mesh] or “ISOFLURANE”[Mesh] or “PROPOFOL”[Mesh] |
15 | Search (halothane*[tiab] or enflurane*[tiab] or isofulrane*[tiab] or sevoflurane*[tiab] or desflurane*[tiab] or propofol*[tiab]) |
16 | Search (UQT9G45D1P[rn] or 91I69L5AY5[rn] or CYS9AKD70P[rn] or 38LVP0K73A[rn] or CRS35BZ94Q [rn] or YI7VU623SF[rn]) |
17 | Search #11 OR #12 OR #13 OR #14 OR #15 OR #16 |
18 | Search #10 AND #17 |
19 | Search (“2017/05/01”[Date - Publication] : “3000”[Date - Publication]) |
20 | Search #18 AND #19 |
Rights and permissions
About this article
Cite this article
Yap, A., Lopez-Olivo, M.A., Dubowitz, J. et al. Anesthetic technique and cancer outcomes: a meta-analysis of total intravenous versus volatile anesthesia. Can J Anesth/J Can Anesth 66, 546–561 (2019). https://doi.org/10.1007/s12630-019-01330-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-019-01330-x