Abstract
Purpose
Dexamethasone prolongs the duration of interscalene block, but the benefits of higher doses and perineural vs intravenous administration remain unclear.
Methods
This factorial design, double-blinded trial randomized 280 adult patients undergoing ambulatory arthroscopic shoulder surgery at a single centre in a 1:1:1:1 ratio. Patients received ultrasound-guided interscalene block with 30 mL 0.5% bupivacaine and 4 mg or 8 mg dexamethasone by either the perineural or intravenous route. The primary outcome (block duration measured as the time of first pain at the surgical site) and secondary outcomes (adverse effects, postoperative neurologic symptoms) were assessed by telephone. In this superiority trial, the predetermined minimum clinically important difference for comparisons between doses and routes was 3.0 hr.
Results
The perineural route significantly prolonged the mean block duration by 2.0 hr (95% confidence interval [CI], 0.4 to 3.5 hr; P = 0.01), but 8 mg of dexamethasone did not significantly prolong the mean block duration compared with 4 mg (1.3 hr; 95% CI, −0.3 to 2.9 hr, P = 0.10), and there was no significant statistical interaction (P = 0.51). The mean (95% CI) block durations, in hours, were 24.0 (22.9 to 25.1), 24.8 (23.2 to 26.3), 25.4 (23.8 to 27.0), and 27.2 (25.2 to 29.3) for intravenous doses of 4 and 8 mg and perineural doses of 4 and 8 mg, respectively. There were no marked differences in side effects between groups. At 14 postoperative days, 57 (20.4%) patients reported neurologic symptoms, including dyspnea and hoarseness. At six months postoperatively, only six (2.1%) patients had residual symptoms, with four (1.4%) patients’ symptoms unlikely related to interscalene block.
Conclusion
Compared with the intravenous route, perineural dexamethasone prolongs the mean interscalene block duration by a small amount that may or may not be clinically significant, regardless of dose. However, the difference in mean block durations between 8 mg and 4 mg of dexamethasone is highly unlikely to be clinically important, regardless of the administration route.
Trial registration
www.clinicaltrials.gov (NCT02426736). Registered 14 April 2015.
Résumé
Objectif
La dexaméthasone prolonge la durée des blocs interscaléniques, mais les avantages de doses plus élevées et d’une administration périnerveuse vs intraveineuse demeurent incertains.
Méthode
Cette étude à double insu et avec plan factoriel a aléatoirement alloué 280 patients adultes devant subir une chirurgie ambulatoire d’arthroscopie de l’épaule dans un seul centre en 4 groupes à un ratio de 1:1:1:1. Les patients ont reçu un bloc interscalénique échoguidé réalisé à l’aide de 30 mL de bupivacaïne 0,5 % et 4 mg ou 8 mg de dexaméthasone, par voie périnerveuse ou intraveineuse. Le critère d’évaluation principal (durée du bloc mesurée au moment de la première douleur au site chirurgical) et les critères d’évaluation secondaires (effets indésirables, symptômes neurologiques postopératoires) ont été évalués par entretien téléphonique. Dans cette étude de supériorité, une différence minimale de 3,0 h a été prédéterminée comme étant cliniquement importante pour comparer les doses et les voies d’administration.
Résultats
La durée moyenne du bloc a été significativement prolongée lors du recours à une voie d’administration périnerveuse, soit de 2,0 h (intervalle de confiance [IC] 95 %, 0,4 à 3,5 h; P = 0,01), mais une dose de 8 mg de dexaméthasone n’a pas prolongé de manière significative la durée moyenne du bloc comparativement à une dose de 4 mg (1,3 h; IC 95 %, −0,3 à 2,9 h, P = 0,10). En outre, aucune interaction statistique significative n’a été observée (P = 0,51). Les durées moyennes (IC 95 %) des blocs, en heures, étaient de 24,0 (22,9 à 25,1), 24,8 (23,2 à 26,3), 25,4 (23,8 à 27,0), et 27,2 (25,2 à 29,3) pour les doses intraveineuses de 4 et 8 mg et les doses périnerveuses de 4 et 8 mg, respectivement. Aucune différence marquée n’a été observée entre les groupes en matière d’effets secondaires. À 14 jours postopératoires, 57 (20,4 %) patients ont rapporté des symptômes neurologiques, y compris la dyspnée et l’enrouement. À six mois postopératoires, seuls six (2,1 %) patients ont mentionné souffrir de symptômes résiduels, et chez quatre (1,4 %) patients, les symptômes n’étaient probablement pas reliés au bloc interscalénique.
Conclusion
Par rapport à une voie d’administration intraveineuse, la dexaméthasone administrée par voie périnerveuse prolonge un peu la durée moyenne du bloc interscalénique, ce qui pourrait ou non avoir une importance clinique, quelle que soit la dose. Toutefois, la différence de durée moyenne des blocs entre des doses de 8 mg et de 4 mg de dexaméthasone n’a probablement pas d’importance clinique, indépendamment de la voie d’administration.
Enregistrement de l’étude
www.clinicaltrials.gov (NCT02426736). Enregistrée le 14 avril 2015.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Arthroscopic shoulder surgery is associated with significant postoperative pain,1 and single-injection interscalene brachial plexus block (ISB) is well established as a preferred analgesic modality for this common ambulatory orthopedic procedure.1,2 Interscalene brachial plexus block can provide reliable surgical anesthesia in addition to superior postoperative analgesia and reduced opioid-related side effects, including postoperative nausea and vomiting.2 Unfortunately, the relatively short duration of analgesia and associated benefits when ISB is performed with local anesthetic alone are a recognized limitation of this technique.2
Among the many adjuncts to perineural local anesthesia that have been investigated to prolong the analgesic duration of ISB,3,4 dexamethasone shows the most promise. Almost without exception, it has significantly prolonged analgesic duration compared with controls when administered by either the intravenous or perineural route.5,6,7,8,9 For patients given ISB for shoulder arthroscopy, however, the optimum route and dose for dexamethasone remain unclear. In this population, studies comparing the same dexamethasone dose given by both the perineural and intravenous routes have shown differences in analgesic duration of one to 4.5 hr. These studies, however, were inadequately powered to precisely estimate the treatment effect,8,10,11 and only one reached statistical significance.6 Three recently published studies have also examined a wide range of dexamethasone doses given by either the perineural9 or intravenous7,12 route. These studies established that doses as low as 2.5 mg could significantly prolong analgesia compared with placebo, but they were underpowered to detect significant differences between lower and higher dexamethasone doses. Also, the results were not consistent with those from an earlier study.13 Direct comparison of results between these studies is not possible because of differences in the definition of analgesic duration, the type of anesthetic, the use of co-analgesics, and the type, dose, and volume of local anesthetic.
To better define the optimum route and dose of dexamethasone for ISB in patients undergoing arthroscopic shoulder surgery, we conducted a factorial designed, double-blinded, randomized, controlled superiority trial comparing lower and higher doses of dexamethasone administered by either the perineural or intravenous route. We hypothesized that the perineural route and higher dexamethasone doses would prolong the mean block duration, without a significant synergistic statistical interaction.
Methods
This two-by-two factorial design study14 was registered (NCT02426736) and approved by the University of Manitoba Biomedical Research Ethics Board (B2015:016, April 1, 2015) and the Winnipeg Regional Health Authority Research Access and Approval Committee (2015-023, June 19, 2015). Health Canada (HC2015:001, February 20, 2015) provided a No Objection Letter granting permission for the perineural use of dexamethasone. The study was conducted at the Pan Am Clinic Surgical Centre, a free-standing ambulatory surgical centre operating within a single-payer, provincially administered health insurance program and staffed by orthopedic surgeons who also operate at inpatient hospitals. Patients scheduled for ambulatory arthroscopic shoulder surgery who were at least 18 yr of age were approached by telephone one week prior to surgery to assess their interest in the study and to screen for eligibility. Interested patients were provided with a consent form by postal or electronic mail. Informed consent was obtained on the day of surgery by research staff blinded to the allocation sequence (M.C., C.F., E.C.), and their eligibility was confirmed by the attending anesthesiologist. Exclusion criteria were the presence of diabetes mellitus, pregnancy, coagulopathy (i.e., international normalized ratio > 1.5), sensitivity to bupivacaine or dexamethasone, severe chronic obstructive pulmonary disease, vocal cord or diaphragmatic paralysis, brachial plexus neuropathy, systemic glucocorticoids during the previous two weeks, epidural or intra-articular corticosteroid injections during the previous three months, daily opioid use during the previous two weeks, active peptic ulcer disease, end-stage renal disease, cirrhotic liver disease, and/or previous participation in the study.
An offsite co-investigator (L.G.), who was otherwise uninvolved with study procedures, created and maintained the computer-generated random allocation sequence (GraphPad Prism version 6.04 for Windows, GraphPad Software, La Jolla CA, USA). The patients were block randomized into groups of 20, thereby allocating patients to one of four study groups in a 1:1:1:1 ratio. Patients received 4 mg or 8 mg of preservative-free, non-particulate dexamethasone sodium phosphate injection USP (Dexamethasone Omega Unidose, 10 mg·mL−1, Omega Laboratories Limited, Montreal, QC, Canada) by either the perineural or intravenous route. On site, the active randomization block information was concealed in a locked drawer and accessed only by F.F. to implement the sequence by privately preparing the study solutions. As an anesthesia clinical assistant, F.F. helped with the block performance and other aspects of the anesthesia under the direction of the attending anesthesiologist but did not assist in postoperative care or the assessment of outcomes.
An ISB solution and an intravenous solution that were indistinguishable, regardless of the intervention arm, were prepared for each patient. The ISB solution contained 30 mL of preservative-free 0.5% bupivacaine (Hospira Healthcare Corporation; Montreal, QC, Canada) and 8 mg dexamethasone, 4 mg dexamethasone, and 0.4 mL normal saline (or, for the intravenous study groups, 0.8 mL normal saline). Similarly, 0.8 mL of normal saline (perineural groups), 4 mg dexamethasone, and 0.4 mL normal saline (or 8 mg of dexamethasone) was added to a 100-mL bag of normal saline to prepare the intravenous solutions. We attempted to blind participants, physicians, recovery room staff, and outcome assessors about these measures.
Preoperatively, with the intravenous solution infusion in place, in-plane ISB was performed using a 22G 50 mm ultrasound needle (Pajunk UniPlex NanoLine; Geisingen Germany) and a 10-5 MHZ linear array transducer (FujiFilm Sonosite; Bothwell, UT, USA). The fifth, sixth, and seventh cervical nerve roots were visualized in an oblique axial plane, and the needle was directed from a posterolateral insertion point.15 The entire ISB solution was then injected incrementally, with frequent aspiration, to surround the nerve roots. Anesthesiologists recorded the time the ISB was completed and noted any adverse events. They were also told to complete the infusion of the intravenous solution prior to surgical incision. All other aspects of perioperative care were at the discretion of the attending anesthesiologist, except that no other agents could be added to the ISB solutions and no corticosteroids administered other than the study drugs.
Once the surgical centre criteria were met postoperatively, patients were discharged home and instructed to take postoperative analgesics as per the surgeon’s preference. Primary and secondary outcomes were assessed by telephone on postoperative day (POD) 1. If necessary, patients were called again on PODs 2 and 3 to establish block duration (the primary outcome). On POD 14, telephone calls were made to assess for postoperative neurologic symptoms (PONS) (Appendix). Patients with symptoms at 14 days were to be contacted at approximately six months postoperatively to assess for symptom resolution.
The primary outcome was defined as the duration of ISB analgesia, measured from the time of completion of the injection of the ISB solution to the time the patient first experienced shoulder pain after surgery, rounded to the nearest 0.1 hr. Secondary outcomes related to analgesia included block failure, defined as requiring opioid analgesia for pain at the surgical site in the recovery room, pain at the time the primary outcome occurred, satisfaction with postoperative analgesia (both assessed by an 11-point numerical rating score, and the cumulative postoperative analgesic consumption at discharge from the recovery room and from discharge until the time the primary outcome occurred). Other secondary outcomes measured by the numerical rating score on POD 1 included postoperative nausea and vomiting, sleep quality on the first postoperative night, dyspnea, restlessness/anxiety, and distress from motor and sensory blockade of the distal arm. We also documented the use of intraoperative vasopressors, antimuscarinic agents, antihypertensive agents, seizures, systemic local anesthetic toxicity, pneumothorax, epidural spread of local anesthetic, recovery room length of stay, unplanned admission to hospital, and PONS (hoarseness, dyspnea, surgical arm numbness or paresthesia, weakness of the hand or fingers).
Statistical analysis
Power analyses were performed with PROC GLMPOWER of SAS version 9.3 (SAS Institute, Cary, NC, USA). With a two-tailed alpha error of 0.05 and a standard deviation of 5.0 hr10,11,13,16 in each group, it was determined that 268 patients would provide 90% power to detect a synergistic interaction of 4.0 hr between dose and route and > 90% power to detect a difference of 3.0 hr in mean block duration for the main effects of dose and route. We considered this 3.0-hr difference to represent a minimum for clinical significance based on an expected analgesic duration of approximately 20 hr.10,11,13,16 Altogether, 280 patients were recruited to account for attrition. After 100 patients completed primary outcome assessments, we carried out a preplanned analysis of only the standard deviations of each randomization group, blinded to the group intervention. The resulting standard deviations of 4.8, 5.4, 5.5, and 7.8 hr were deemed close enough to the 5.0 hr projected in the power calculations, such that recruiting to the projected sample size of 280 would not be futile for assessing the statistical interaction between dose and route.
The primary outcome was first analyzed according to a modified intention-to-treat principle, excluding only those patients who did not undergo an attempt at ISB (i.e., withdrew or were withdrawn between randomization and the ISB attempt). A preplanned secondary analysis of the primary outcome excluded patients who had a failed ISB as defined above, and a preplanned tertiary analysis was a multivariable analysis of the primary outcome. These analyses of the mean block duration were conducted with linear regression models, with the 4-mg intravenous group considered the reference group. An interaction term between dose and route was investigated in the primary and secondary analyses along with their main effects. Variables were selected for the multivariable analysis based on a statistically significant (P < 0.05) relation with the primary outcome in univariate tests. In all models, residual normality was graphically explored with QQ plots, histograms, and scatterplots of residuals against the model’s predicted value. Linearity was assessed with scatterplots of residuals against continuous predictors. Cook’s distance was used to identify influential observations. Other secondary outcome analyses were conducted using Pearson’s Chi-square, Fisher’s exact, or Kruskal–Wallis tests, as appropriate. All analyses were made with SAS version 9.3 (SAS Institute, Cary NC, USA).
Results
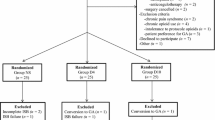
Altogether, 280 patients were enrolled from 674 assessed for eligibility between June 25, 2015 and July 12, 2016 (Fig. 1). All randomized patients had an ISB and provided complete data sets, except for 25 patients for whom postoperative analgesic use could not be calculated. Eight surgeons participated in the study (treating 10-93 patients each) with 40 attending anesthesiologists either performing the ISB directly or via a supervised anesthesiology resident. Eight anesthesiologists performed more than ten ISBs for this study, and 18 performed fewer than five. Patients, procedures, and perioperative care were similar between groups (Table 1). One protocol violation occurred wherein an additional 8 mg of intravenous dexamethasone was given to a patient in the 4-mg intravenous group who subsequently reported 22.0 hr of analgesia.
The initial primary outcome models did not identify a significant statistical interaction between dose and route in either the intention-to-treat analysis (1.0 hr; 95% confidence interval [CI], −2.1 to 4.2 hr, P = 0.51) or when four failed blocks were excluded (0.9 hr; 95% CI, −2.3 to 4.0 hr, P = 0.59). Because of the statistical insignificance and the small estimated size of the interaction term, all subsequent analyses were performed without an interaction term in the model. In these factorial analyses, the perineural route significantly prolonged the mean (95% CI) block duration by 2.0 (0.4 to 3.5) hr (P = 0.01), with the 95% CI still including the 3.0 hr predetermined threshold for clinical significance (Table 2, Fig. 2). The 8-mg dose of dexamethasone, however, did not significantly prolong the mean block duration compared with 4 mg (1.3 hr; 95% CI, −0.3 to 2.9 hr; P = 0.10). It is thus highly unlikely to prolong block duration by a clinically significant amount. Exclusion of four failed blocks did not change this interpretation for the perineural (2.1 hr; 95% CI, 0.6 to 3.7 hr; P = 0.008) or 8 mg doses (1.4 hr; 95% CI, −0.2 to 3.0 hr; P = 0.08).
Recovery room time (from arrival to discharge home, expressed as the median [interquartile range]) with the 4-mg dose (1.6 [1.4-1.8] hr was significantly different (P < 0.001) from that with the 8-mg dose (1.4 [1.3-1.7] hr). Conversely, recovery room time with the perineural route (1.5 [1.3-1.8] hr) was not significantly different (P = 0.07) from that with the intravenous route (1.5 [1.3-1.8] hr). The other secondary outcomes also were not statistically different (Table 2).
A model predicting block duration was created from those characteristics that were significant predictors (P < 0.10) of mean block duration (Table 3). Notably, several possible confounders of patient-perceived block duration that were not standardized in this pragmatic study were not significant predictors. Thus, local anesthetic infiltration of the posterior port site, the use of general anesthesia, and the administration of any opioid intraoperatively or in the recovery room are not included in this multivariable analysis. The model results were sensitive to an outlier with an unusually long block duration (58.8 hr). The predictive power of the model was weak with (R2 = 0.15) or without (R2 = 0.17) inclusion of the outlier in the model.
Six patients experienced complications related to block performance (P = 0.21 for dose; P = 0.68 for route), none of whom had postoperative neurologic symptoms at POD 14. Four patients in the 8-mg perineural group and one patient in the 8-mg intravenous group experienced transient paresthesias. The sixth patient had block-related pneumothorax and was admitted to hospital for observation. One other patient given 4 mg perineural dexamethasone attended an emergency room for several hours due to shortness of breath several hours after discharge from the surgical centre.
At POD 14, five patients reported dyspnea, three of whom had been given alternative diagnoses of constipation, worsening sarcoidosis confirmed on imaging, and asthma exacerbation due to tobacco smoke exposure. An additional five had a hoarse voice, and two reported both hoarse voice and dyspnea. None of these 12 patients had concomitant PONS in the surgical arm, and only one patient had a persistently hoarse voice at the six-month follow up. In comparison, 45 (16%) patients reported PONS in the surgical arm at POD 14. The incidence tended to be numerically higher at the higher dose and in the perineural route groups but did not reach statistical significance. Among the six patients who had persistent symptoms at the six-month follow-up, the relation to ISB seemed coincidental in four. In one other patient, preexisting neurologic disease was likely a contributing factor (Table 4).
Discussion
We found that, when single-injection interscalene block with 30 mL bupivacaine and dexamethasone is used for patients undergoing ambulatory arthroscopic shoulder surgery, the perineural route prolongs the mean block duration by < 10% of the mean block duration obtained using the same dose of intravenous dexamethasone. We found no significant difference in effectiveness between dexamethasone doses of 8 mg and 4 mg, despite being better powered than previous studies7,9,12,13 to do so. We also found no marked differences in various side effects measured as secondary outcomes, and no statistically significant interaction between dose and route. These single-centre study results should be applicable to other patient populations given the large number of surgeons and anesthesiologists involved in the study and the pragmatic design, whereby caregivers continued usual practice and patients took oral analgesics as required. We also chose a primary outcome—first shoulder pain detected following the anesthesia and surgery—that we believe is less subjective, more patient-centered and more compatible with typical postoperative multimodal analgesic regimens than the first analgesic request,6,7,9,10,12 first analgesic administration,11 or complete resolution of the shoulder sensory blockade.8
One advantage of the two-by-two group factorial design used in this study is the opportunity to test for a statistical interaction,14 which in the context of this study could be conceptualized as a difference in the slope of the dexamethasone dose-response curve between the two routes of administration (perineural vs intravenous). A synergistic interaction between the 8-mg dose and the perineural route would mean that the increase in mean block duration in the 8-mg perineural group vs the 4-mg perineural group was much larger than the increase in mean block duration in the 8-mg intravenous group vs the 4-mg intravenous group. To our knowledge, this study is the first to attempt to measure this parameter in this population. The estimated value, 1.0 hr [95% CI, -2.1 to 4.2 hr], suggests that a clinically important interaction between dose and route is unlikely. Conversely, a clinically small interaction (synergistic or antagonistic) cannot be ruled out based on our results. If an interaction truly does exist, not accounting for its presence in the analysis increases the chance of type I error for estimating the main effects of dose and route.17 The empirical effect on the study results would depend on the type of interaction (synergistic or antagonistic). A synergistic interaction—predicted to be most likely in the model—would result in overestimating the separate main effects of the perineural route and 8-mg dose, which in reality would be partly dependent on specifically using the perineural route with the 8-mg dose (i.e., the interaction term effect). An antagonistic interaction (i.e., the interaction term is negative)—also plausible based on the confidence intervals for the interaction term and not accounting for its presence in the analysis—would have the opposite effect. Importantly, the magnitude of these biases, equal to one-half the magnitude of the interaction term,17 are likely not clinically important given the relatively small size of the plausible values for the interaction term. Finally, even if there was truly no interaction, a small non-zero interaction term in the model would be expected based on random sampling error. Unfortunately, a very large increase in study size is necessary for a precise estimate of a small interaction term.14 The costs of such a study would need to be justified by a significant added clinical value for better understanding the relations between the dexamethasone dose, route, and mean block duration. We do not believe such justification currently exists, given the relatively small effects of dose and route and the large amount of residual unexplained variability in mean block duration in the multivariable model, despite the model being developed from a wide array of patient-, surgery-, and anesthesia-related predictor variables.
With neither a clinically nor statistically significant interaction term, we have interpreted our results in the context of the main effects only.14 In that regard, the study was powered and interpreted based on a three-hour difference between groups being a threshold for clinical significance. As this threshold is subjective, clinicians should consider their values and those of their patients when applying the study findings.
A second advantage of the two-by-two factorial design is increased statistical power to study the main effects of dose and route, compared with a study of the same size analyzed as four separate groups.14 Consequently, this study has been able to estimate the effects of the dexamethasone dose and route on ISB duration more precisely than in previous work. Two published perineural vs intravenous comparisons of 8 or 10 mg dexamethasone and ropivacaine 0.5% (28 or 30 mL) concluded that there was no significant difference in analgesic duration, but they were underpowered to do so.8,11 Rosenfeld et al.,8 found a nonsignificant decrease in mean block duration with the perineural route (-1.3 hr; 95% CI, -4.0 to 1.2 hr), whereas Desmet et al.11 found a nonsignificant increase in median block duration with the perineural route (2.2 hr; P = 0.63). In contrast, two other similarly powered studies using 4 or 5 mg of dexamethasone and ropivacaine 0.5% (28 or 12 mL)6,10 found that the perineural route significantly6 prolonged the median block duration over the intravenous route by 4.0-4.5 hr, or 25%. These results, together with another underpowered study suggesting that 8 mg of perineural dexamethasone might substantially prolong the mean block duration over 4 mg,13 inspired the factorial design for this study. We have found that when bupivacaine is used, regardless of the dexamethasone dose (4 or 8 mg), perineural dexamethasone significantly prolongs the mean block duration compared with intravenous dexamethasone (2.0 hr; 95% CI, 0.4 to 3.5 hr; P = 0.01). The calculated 95% confidence intervals, corresponding to approximately 2% to 15% relative increases in mean block duration, indicate that the true effect may or may not be clinically significant. Previous study results likely represent inadequate statistical power,8,11,13 random sampling error, different primary outcome measurements, or effects idiosyncratic to ropivacaine.6,10
We chose to use bupivacaine for our study because it was already in common use at our facility, and it would likely provide a longer block duration than ropivacaine, potentially offering a pain-free first postoperative night for most patients.13,18 We respectively chose 8-mg and 4-mg doses of dexamethasone as the highest dose typically used in local practice and the lowest dose in the literature published at the time the study was being designed (4-10 mg). Two recent studies found that doses as low as 2.5 mg, administered by either the perineural9 or intravenous7 route, prolonged ISB duration vs placebo, but neither study compared the duration of ISB analgesia for different dexamethasone doses. Chalifoux et al. recently determined that a 10-mg intravenous dexamethasone dose did not significantly prolong the median block duration when compared with a 4-mg dose (median difference, 0.5 hr; 95% CI, -2.8 to 3.7 hr).12 In this larger study, we found that 8 mg did not significantly increase the mean block duration (1.3 hr; 95% CI -0.3 to 2.9 hr) compared with 4 mg, and that it is very unlikely that it would prolong the block duration by > 3.0 hr, regardless of the administration route. We cannot completely rule out, however, a slight prolongation of block duration with the 8-mg dose over the 4-mg dose, with either the intravenous or perineural route.
We chose not to attempt, for practical reasons, to measure the systemic adverse effects of dexamethasone. Meta-analyses have suggested that the effect of a single perioperative dose of dexamethasone in the range used herein has minimal effect on blood glucose levels and no adverse effects on wound healing or infection rates.19 To minimize the potential effect of these changes, we excluded patients at the highest risk of corticosteroid-related adverse effects, so the results may not be generalizable to this and other excluded patient populations. We also did not measure a standardized quality of recovery score that might have identified additional differences between groups or related the dexamethasone dose and route to overall quality of recovery. Our secondary outcomes, however, which provided no sign of between-group differences, examined many of the items in the emotional and comfort domains of a popular scale,20 and our primary outcomes overlapped with the pain domain. The remaining domains, physical independence and patient support, are much less likely to be affected by the dexamethasone dose or administration route than by surgical centre processes, preexisting patient factors, type of surgery, and anesthetic.
The surgical arm PONS rates of 16% at 14 days and 1.8% at six months in this study are higher than those reported in large, recent prospective studies of PONS after ultrasound-guided ISB without dexamethasone as an adjuvant.21,22 We suspect several factors are implicated in this finding. Our assessment tool (see Appendix) was completely inclusive even for transient distal ulnar, median, or other paresthesias due to postoperative sling positioning or prolonged immobility. We did not exclude nerve injuries presumed to be of surgical etiology, which have been reported in up to 10% of patients in other series.23 In contrast, the use of a baseline neurologic examination,21 and the exclusion of cases that the authors determined were unrelated to the ISB by clinical examination21,22 would be expected to reduce substantially the reported rates in those series.23 In the current study, almost all cases of PONS at POD 14 resolved by six months after surgery, as occurred in other studies with similar, higher rates of early PONS.24,25 Notably, when the exclusion criteria of the large series are applied to our cases of PONS at the six-month follow-up, one case would be excluded for an abnormal baseline examination and four others for symptoms unrelated to ISB, leaving one of 280 patients. This PONS rate of 0.4% at six months is similar to what was reported in those studies.21,22
That dexamethasone could contribute to an increased incidence of PONS should be considered. Despite a long track record of safety recorded in the chronic pain literature,26 there has been considerable concern regarding the safety of administering preservative-free dexamethasone by the perineural route.27 Local anesthetic adjuvants have not been investigated as covariates in recent medium or large series of PONS. 21,22,24,25 The incidence of PONS at POD 14 in this study ranged from 9% in the 4-mg intravenous dexamethasone group to 23% in the 8-mg perineural group. Differences between the two routes and doses did not reach statistical significance, although our study was not powered to assess this parameter adequately. Remarkably, three previously published trials of ISB with dexamethasone6,28,29 did not report PONS at all. The remainder5,7,8,9 did not pre-specify this outcome in a trial registry, raising concerns about the rigor with which cases were ascertained.
We ultimately interpreted our findings as demonstrating that perineural dexamethasone does not offer a significant clinical advantage over intravenous dexamethasone for ISB. Until the safety of the perineural route is established more definitively, it is difficult to justify the routine use of perineural dexamethasone in ISB for outpatient shoulder surgery. Given that a clinically significant benefit of > 3.0 hr with the use of 8 mg of dexamethasone compared with 4 mg is highly unlikely based on our results, we favor 4 mg of intravenous dexamethasone in this population as an effective analgesic dose with an established safety record. Future research should focus on characterizing and minimizing PONS in patients receiving ISB for shoulder surgery and on comparing postoperative analgesia between patients receiving 4 mg of intravenous dexamethasone with other adjuncts.30
References
Hughes MS, Matava MJ, Wright RW, Brophy RH, Smith MV. Interscalene brachial plexus block for arthroscopic shoulder surgery: a systematic review. J Bone Joint Surg Am 2013; 95: 1318-24.
Abdallah FW, Halpern SH, Aoyama K, Brull R. Will the real benefits of single-shot interscalene block please stand up? A Systematic review and meta-analysis. Anesth Analg 2015; 120: 1114-29.
Brummett CM, Williams BA. Additives to local anesthetics for peripheral nerve blockade. Int Anesthesiol Clin 2011; 49: 104-16.
Kirksey MA, Haskins SC, Cheng J, Liu SS. Local anesthetic peripheral nerve block adjuvants for prolongation of analgesia: a systematic qualitative review. PLoS One 2015; 10: e0137312.
Albrecht E, Kern C, Kirkham KR. A systematic review and meta-analysis of perineural dexamethasone for peripheral nerve blocks. Anaesthesia 2015; 70: 71-83.
Chun EH, Kim YJ, Woo JH. Which is your choice for prolonging the analgesic duration of single-shot interscalene brachial blocks for arthroscopic shoulder surgery? Intravenous dexamethasone 5 mg vs. perineural dexamethasone 5 mg randomized, controlled, clinical trial. Medicine (Baltimore) 2016; 95: e3828.
Desmet M, Vanneste B, Reynvoet M, et al. A randomised controlled trial of intravenous dexamethasone combined with interscalene brachial plexus blockade for shoulder surgery. Anaesthesia 2015; 70: 1180-5.
Rosenfeld DM, Ivancic MG, Hattrup SJ, et al. Perineural versus intravenous dexamethasone as adjuncts to local anaesthetic brachial plexus block for shoulder surgery. Anaesthesia 2016; 71: 380-8.
Woo JH, Kim YJ, Kim DY, Cho S. Dose-dependency of dexamethasone on the analgesic effect of interscalene block for arthroscopic shoulder surgery using ropivacaine 0.5%: a randomised controlled trial. Eur J Anaesthesiol 2015; 32: 650-5.
Kawanishi R, Yamamoto K, Tobetto Y, et al. Perineural but not systemic low-dose dexamethasone prolongs the duration of interscalene block with ropivacaine: a prospective randomized trial. Local Reg Anesth 2014; 7: 5-9.
Desmet M, Braems H, Reynvoet MP, et al. I.V. and perineural dexamethasone are equivalent in increasing the analgesic duration of a single-shot interscalene block with ropivacaine for shoulder surgery: a prospective, randomized, placebo-controlled study. Br J Anaesth 2013; 111: 445-52.
Chalifoux F, Colin F, St-Pierre P, Godin N, Brulotte V. Low dose intravenous dexamethasone (4 mg and 10 mg) significantly prolongs the analgesic duration of single-shot interscalene block after arthroscopic shoulder surgery: a prospective randomized placebo-controlled study. Can J Anesth 2017; 64: 280-9.
Tandoc MN, Fan L, Kolesnikov S, Kruglov A, Nader ND. Adjuvant dexamethasone with bupivacaine prolongs the duration of interscalene block: a prospective randomized trial. J Anesth 2011; 25: 704-9.
Montgomery AA, Peters TJ, Little P. Design, analysis and presentation of factorial randomised controlled trials. BMC Med Res Methodol 2003; 3: 26.
Soeding P, Eizenberg N. Review article: anatomical considerations for ultrasound guidance for regional anesthesia of the neck and upper limb. Can J Anesth 2009; 56: 518-33.
Cummings KC 3rd, Napierkowski DE, Parra-Sanchez I, et al. Effect of dexamethasone on the duration of interscalene nerve blocks with ropivacaine or bupivacaine. Br J Anaesth 2011; 107: 446-53.
Kahan BC. Bias in randomised factorial trials. Stat Med 2013; 32: 4540-9.
Banghu M, Mutter T, Dubberley J, MacDonald P, Dufault B, Amadeo R. Single-injection interscalene bupivacaine and dexamethasone for same-day discharge total shoulder arthroplasty: a case series. Can J Anesth 2017; 64: 435-7.
Waldron NH, Jones CA, Gan TJ, Allen TK, Habib AS. Impact of perioperative dexamethasone on postoperative analgesia and side-effects: systematic review and meta-analysis. Br J Anaesth 2013; 110: 191-200.
Gornall BF, Myles PS, Smith CL, et al. Measurement of quality of recovery using the QoR-40: a quantitative systematic review. Br J Anaesth 2013; 111: 161-9.
Liu SS, Gordon MA, Shaw PM, Wilfred S, Shetty T, Yadeau JT. A prospective clinical registry of ultrasound-guided regional anesthesia for ambulatory shoulder surgery. Anesth Analg 2010; 111: 617-23.
Sites BD, Taenzer AH, Herrick MD, et al. Incidence of local anesthetic systemic toxicity and postoperative neurologic symptoms associated with 12,668 ultrasound-guided nerve blocks: an analysis from a prospective clinical registry. Reg Anesth Pain Med 2012; 37: 478-82.
Dwyer T, Henry PD, Cholvisudhi P, Chan VW, Theodoropoulos JS, Brull R. Neurological complications related to elective orthopedic surgery: part 1: common shoulder and elbow procedures. Reg Anesth Pain Med 2015; 40: 431-42.
Borgeat A, Ekatodramis G, Kalberer F, Benz C. Acute and nonacute complications associated with interscalene block and shoulder surgery: a prospective study. Anesthesiology 2001; 95: 875-80.
Liu SS, Zayas VM, Gordon MA, et al. A prospective, randomized, controlled trial comparing ultrasound versus nerve stimulator guidance for interscalene block for ambulatory shoulder surgery for postoperative neurological symptoms. Anesth Analg 2009; 109: 265-71.
MacMahon PJ, Huang AJ, Palmer WE. Spine injectables: what is the safest cocktail? AJR Am J Roentgenol 2016; 207: 526-33.
Noss CD, MacKenzie LD, Kostash MA. Adjuvant dexamethasone: innovation, farce, or folly? Reg Anesth Pain Med 2014; 39: 540-5.
Vieira PA, Pulai I, Tsao GC, Manikantan P, Keller B, Connelly NR. Dexamethasone with bupivacaine increases duration of analgesia in ultrasound-guided interscalene brachial plexus blockade. Eur J Anaesthesiol 2010; 27: 285-8.
Kim YJ, Lee GY, Kim DY, Kim CH, Baik HJ, Heo S. Dexamathasone added to levobupivacaine improves postoperative analgesia in ultrasound guided interscalene brachial plexus blockade for arthroscopic shoulder surgery. Korean J Anesthesiol 2012; 62: 130-4.
Abdallah FW, Dwyer T, Chan VW, et al. IV and perineural dexmedetomidine similarly prolong the duration of analgesia after interscalene brachial plexus block: a randomized, three-arm, triple-masked, placebo-controlled trial. Anesthesiology 2016; 124: 683-95.
Acknowledgements
This research was supported by the Canadian Anesthesia Research Foundation, the Pan Am Clinic Foundation and the University of Manitoba Department of Anesthesia and Perioperative Medicine Academic Oversight Committee.
Conflicts of interest
None declared.
Editorial responsiblity
This submission was handled by Dr. Gregory L. Bryson, Deputy Editor-in-Chief, Canadian Journal of Anesthesia.
Author contributions
Darren Holland contributed to study design, acquisition, analysis, and interpretation of data, drafted the manuscript, critically revised it, and approved the final version. Ryann J.J. Amadeo, Scott Wolfe, Faylene Funk, and Mark Collister contributed to study design, acquisition of data, critically revised the manuscript, and approved the final version. Linda Girling, Jeff Leiter, Jason Old, and Peter MacDonald contributed to study design, critically revised the manuscript, and approved the final version. Emily Czaplinski and Celeste Ferguson contributed to data acquisition, critically revised the manuscript, and approved the final version. Brenden Dufault contributed to study design, analysis, and interpretation of data, critically revised the manuscript and approved the final version. Thomas C. Mutter contributed to study design, analysis, and interpretation of the data, revised the manuscript, and approved the final version.
Funding
This research was supported by the Canadian Anesthesia Research Foundation, the University of Manitoba Department of Anesthesia and Perioperative Medicine Academic Oversight Committee, and the Pan Am Clinic Foundation.
Author information
Authors and Affiliations
Corresponding author
Appendix: Postoperative day 14 phone call assessment questions
Appendix: Postoperative day 14 phone call assessment questions
-
1)
Have you experienced any of the following:
-
a)
Hoarse voice that has lasted since the surgery was performed? Yes/No
-
b)
Shortness of breath that has lasted since the surgery was performed? Yes/No
-
a)
-
2)
On the side where the surgery was performed, are you currently experiencing any of the following in the shoulder, arm, or hand?
-
a)
Numbness? Yes/No
-
b)
Tingling sensation? Yes/No
-
c)
Weakness of the hand or fingers? Yes/No
-
a)
Rights and permissions
About this article
Cite this article
Holland, D., Amadeo, R.J.J., Wolfe, S. et al. Effect of dexamethasone dose and route on the duration of interscalene brachial plexus block for outpatient arthroscopic shoulder surgery: a randomized controlled trial. Can J Anesth/J Can Anesth 65, 34–45 (2018). https://doi.org/10.1007/s12630-017-0989-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-017-0989-7