Abstract
Background
Previously active in the mid-1990s, the Canadian Airway Focus Group (CAFG) studied the unanticipated difficult airway and made recommendations on management in a 1998 publication. The CAFG has since reconvened to examine more recent scientific literature on airway management. The Focus Group’s mandate for this article was to arrive at updated practice recommendations for management of the unconscious/induced patient in whom difficult or failed tracheal intubation is encountered.
Methods
Nineteen clinicians with backgrounds in anesthesia, emergency medicine, and intensive care joined this iteration of the CAFG. Each member was assigned topics and conducted reviews of Medline, EMBASE, and Cochrane databases. Results were presented and discussed during multiple teleconferences and two face-to-face meetings. When appropriate, evidence- or consensus-based recommendations were made together with assigned levels of evidence modelled after previously published criteria.
Conclusions
The clinician must be aware of the potential for harm to the patient that can occur with multiple attempts at tracheal intubation. This likelihood can be minimized by moving early from an unsuccessful primary intubation technique to an alternative “Plan B” technique if oxygenation by face mask or ventilation using a supraglottic device is non-problematic. Irrespective of the technique(s) used, failure to achieve successful tracheal intubation in a maximum of three attempts defines failed tracheal intubation and signals the need to engage an exit strategy. Failure to oxygenate by face mask or supraglottic device ventilation occurring in conjunction with failed tracheal intubation defines a failed oxygenation, “cannot intubate, cannot oxygenate” situation. Cricothyrotomy must then be undertaken without delay, although if not already tried, an expedited and concurrent attempt can be made to place a supraglottic device.
Résumé
Contexte
Actif au milieu des années 1990, le Canadian Airway Focus Group (CAFG), un groupe dédié à l’étude des difficultés imprévues dans la prise en charge des voies aériennes, a émis des recommandations sur ce sujet dans une publication datant de 1998. Le CAFG s’est réuni à nouveau pour passer en revue la littérature scientifique récente concernant la prise en charge des voies aériennes. Dans cet article, le CAFG s’est donné pour mission d’émettre des recommandations visant la prise en charge du patient inconscient ou anesthésié qui présente des difficultés d’intubation significatives.
Méthode
Dix-neuf cliniciens ayant une formation en anesthésie, en médecine d’urgence ou en soins intensifs composent le CAFG actuel. Les participants ont passé en revue des sujets précis en consultant les bases de données Medline, EMBASE et Cochrane. Les résultats de ces revues ont été présentés et discutés dans le cadre de téléconférences et de deux réunions en personne. Lorsqu’indiqué, des recommandations fondées sur des données probantes ou sur un consensus ont été émises. Le niveau de confiance attribué à ces recommandations a aussi été défini.
Conclusion
Le clinicien doit avoir conscience des lésions qu’il peut infliger lors de tentatives multiples d’intubation trachéale. Il est possible d’éviter de telles lésions en abandonnant rapidement une technique d’intubation infructueuse afin d’opter pour une méthode alternative (ou ‘plan B’) à condition que l’oxygénation par masque facial ou par l’utilisation d’un dispositif supraglottique s’avère possible. Nonobstant la ou les techniques choisies, un maximum de trois tentatives infructueuses mène à la conclusion qu’il s’agit d’un échec d’intubation et devrait inciter le clinicien à adopter une stratégie de retrait. Une situation dans laquelle il est impossible de procéder à l’oxygénation du patient à l’aide d’un masque facial, d’un dispositif supraglottique ou de l’intubation endotrachéale est qualifiée de scénario cannot intubate, cannot ventilate. Il est alors impératif de procéder sans délai à une cricothyrotomie, à moins que l’insertion d’un dispositif supraglottique n’ait été tentée. Celle-ci peut alors être effectuée rapidement et parallèlement à la réalisation de la cricothyrotomie.
Similar content being viewed by others
What other statements of recommendation are available on this topic?
In 1998, Canadian recommendations were published on management of the unanticipated difficult airway. More recent national recommendations and guidelines on difficult airway management have been published in the USA, the United Kingdom, and other western European countries.
Why were these recommendations developed?
Canadian recommendations were overdue for an update. Since the last review, many new devices useful in difficult airway management have been introduced. In addition, the Anesthesia Closed Claims Project and other observational studies have highlighted potential areas for improvement in management of the difficult and failed airway.
How do these statements differ from existing recommendations?
These statements reflect current evidence and thinking on an appropriate response to difficult airway management encountered in the unconscious/induced patient. The importance of engaging an exit strategy after a limited number of attempts at tracheal intubation is emphasized, as is a simplified response to a failed oxygenation, “cannot intubate, cannot oxygenate” situation.
Why do these statements differ from existing recommendations?
These statements differ from existing recommendations in order to simplify decision-making when failed tracheal intubation or failed oxygenation is encountered in the unconscious/induced patient.
Contents
Methods
Definitions
Incidence and scope of the problem
Management of the difficult and failed airway in the unconscious/induced patient
The primary approach to tracheal intubation: “Plan A”
Response to difficulty encountered in the unconscious patient
Unsuccessful primary approach to tracheal intubation
The alternative approach to tracheal intubation: “Plan B” in the adequately oxygenated patient
Failed tracheal intubation in the adequately oxygenated patient
Limits to attempts at tracheal intubation
Failed intubation: exit strategies
Failed oxygenation: the emergency strategy
Tracheal intubation confirmation
The obstetric airway: special considerations
The pediatric airway: special considerations
Documentation following an encounter with a difficult airway
Education in the management of a difficult airway
Summary of recommendations
References
Appendices
DISCLAIMER: | |
Care has been taken to confirm the accuracy of the information presented and to describe generally accepted practices. The authors accept that medical knowledge is an ever-changing science that continually informs, improves, and alters attitudes, beliefs, and practices. | |
Recommendations are not intended to represent or be referred to as a standard of care in the management of the difficult or failed airway. | |
Application of the information provided in a particular situation remains the professional judgement and responsibility of the practitioner. |
Bedside predictors of difficult tracheal intubation are imperfect. Accordingly, when general anesthesia (GA) is induced despite predictors of difficult intubation, many cases prove unchallenging. Conversely, unanticipated failure of tracheal intubation by direct laryngoscopy or other technique can occur when no such challenges were expected. Encountering difficult tracheal intubation in the unconscious patient is a concern, as many studies involving several specialties have documented increasing patient morbidity with multiple tracheal intubation attempts.1-5
Other hazards associated with difficulty in airway management have been highlighted in recent publications. Studies of closed legal actions6-8 related to airway management and the recent 4th National Audit Project (NAP4) of the Royal College of Anaesthetists and the Difficult Airway Society in the United Kingdom9,10 have helped direct attention to problem areas. In the NAP4 study, a prospective registry was created of major complications related to airway management occurring over a 12-month period in all 309 National Health Service hospitals in the United Kingdom. Complications were reported if they led to death, brain damage, need for emergency surgical airway, unanticipated intensive care unit (ICU) admission, or prolongation of ICU stay.9 The results of the audit provide considerable insight into causes of airway management-related morbidity and potential areas for improvement.
This first of two publications addresses airway management in the unconscious patient when difficult tracheal intubation is encountered. The second publication will focus on options and the approach to the patient when difficult airway management is anticipated.11 Taken together, the articles are intended to assist the practitioner with recommendations for airway management when confronted with a difficult or failed airway, regardless of where in the hospital an airway intervention occurs.
Methods
The Canadian Airway Focus Group (CAFG) was originally formed in the mid-1990s and published recommendations for the management of the unanticipated difficult airway in 1998.12 Four of the original CAFG members rejoined the current iteration, and the first author invited an additional 14 clinicians with an interest in airway management to participate. The current Focus Group includes representatives from anesthesiology, emergency medicine, and critical care.
Topics for review were divided among the members, and participants conducted a literature review on their topic(s). Electronic literature searches were not conducted according to a strict protocol, but participants were instructed to search, at a minimum, Medline and EMBASE databases together with the Cochrane Central Register of Controlled Trials (CENTRAL). Search strings were determined by individual participants. A worksheet was completed for each topic with details of the search strategy, a synopsis of the relevant studies, an overall summary of findings, the perceived quality of evidence, and the author’s suggestion(s) for strength of recommendation (see below). Once finished, worksheets were made available to the CAFG membership on a file hosting service.
The Focus Group convened regularly by teleconference, and face-to-face meetings occurred twice during the 24 months taken to complete the process. Worksheet authors presented their topics to the members, who then arrived at consensus on overall quality of evidence and any recommendations. In the event that evidence was of low quality or altogether lacking, “expert opinion” by consensus was sought. Finally, a draft of the completed manuscript was distributed to all members for review prior to submission.
The strength of a recommendation and the accompanying level of evidence were modelled after the GRADE system, as per previously published criteria.13,14 When made, formal strength of recommendations adhere to the following descriptors:
-
Strong recommendation for – most patients should receive the intervention; most patients in this situation would want the recommended course of action;
-
Weak recommendation for – most patients would want the suggested course of action, but some would not; the appropriate choice may vary for individual patients.
-
Strong recommendation against – most patients should not receive the intervention; most patients in this situation would not want the suggested course of action;
-
Weak recommendation against – most patients would not want the suggested course of action, but some would; the appropriate choice may vary for individual patients.
Three levels of evidence were applied,13 as follows:
-
Level of evidence A (High) – systematic reviews of randomized controlled trials (RCTs), RCTs without important limitations, or observational studies providing overwhelming evidence;
-
Level of evidence B (Moderate) – RCTs with limitations, observational studies with significant therapeutic effect;
-
Level of evidence C (Low) – RCTs with significant limitations, observational studies, case series, or published expert opinion.
When a level of evidence is not specifically supplied in this manuscript, recommendations reflect the consensus opinion of the authors.
Definitions
The following definitions of terms are presented to clarify their use in the text. Some definitions have changed from the 1998 iteration of these recommendations to reflect the increased use of alternatives to direct laryngoscopy (DL) and ventilation with a supraglottic device (SGD).
Difficult airway: A difficult airway can be defined as one where an experienced provider anticipates or encounters difficulty with any or all of face mask ventilation, direct or indirect (e.g., video) laryngoscopy, tracheal intubation, SGD use, or surgical airway.
Difficult face mask ventilation: It has been suggested that inadequate mask ventilation may be more difficult to recognize than its complete absence.15 Although various definitions relating to difficulties with mask ventilation have been proposed,16-18 ease of mask ventilation is best described as a continuum from no difficulty to impossible. Difficult face mask ventilation may be signified by manipulations required for its facilitation, including adjustments of the head and neck, the use of adjuvants (e.g., an oral or nasal airway), use of exaggerated jaw lift, two-handed face mask application, and the assistance of a second operator.
Difficult laryngoscopy: Laryngeal exposure using DL is generally quantified using the Cormack-Lehane grade19 or one of its modifications.20,21 Most authorities agree that grade 1 and 2 views, where most or some portion of the glottis is seen, represent easy DL, while grade 3 and 4 views represent difficult and failed DL, respectively, even if tracheal intubation itself succeeds. The same classification can be employed when indirect techniques, such as video laryngoscopy, are utilized. Regardless of the technique used (DL or indirect laryngoscopy), the specific device should always be described in addition to the view obtained, the number of attempts, and the ancillary maneuvers required to achieve the result.
Difficult tracheal intubation: The success of direct or indirect laryngoscopy and tracheal intubation should be assessed independently, regardless of the technique. Difficult tracheal intubation can be defined as one or all of the following:12
-
Multiple attempts or more than one operator required;
-
An adjunct such as a tracheal tube introducer (“bougie”) is required to facilitate tracheal intubation;
-
An alternative intubation device is required after unsuccessful use of the primary, “Plan A” device.
A common reason for difficulty with tracheal intubation is a poor laryngeal view; however, if a Cormack-Lehane 1 or 2 view is obtained but difficulty occurs with directing or advancing the endotracheal tube (as may happen with video laryngoscopy), it is reasonable to describe this in some form of narrative. Alternatively, difficulty can be quantified using a scale based on several parameters.22
Difficult SGD use: Difficult or failed oxygenation and ventilation with an SGD may result from difficulties accessing the patient’s mouth or hypopharynx, achieving a seal,23 or ventilating the lungs.
Difficult transtracheal surgical airway: A surgical airway can be achieved by percutaneous needle-guided cannula methods or by an open operative technique. A difficult transtracheal surgical airway is one that requires excess time or multiple efforts.
Failed airway: Defining a failed airway helps serve notice to the clinician that a different course of action may be needed to minimize the potential for harm to the patient:
-
Failed tracheal intubation can be defined as failure to achieve successful tracheal intubation in a maximum of three attempts, irrespective of the technique(s) used.
-
Failed oxygenation (“cannot intubate, cannot oxygenate” [CICO])24 has occurred if, in the face of failed tracheal intubation, the patient cannot be successfully oxygenated by employing face mask or SGD ventilation.
Extubation of the difficult airway: Extubation is unsuccessful when a tracheal tube is removed but requires unanticipated replacement. This replacement (including tracheal tube exchange) can be difficult or fail. A clear definition of difficulty does not exist, but it is reasonable to assume that the difficulty further contributes to rather than resolves a deteriorating situation. A high-risk extubation can be described on two axes: the risk of not tolerating extubation and the risk of re-intubation being difficult or unsuccessful. Extubation of the patient with a difficult airway is addressed in the second article in this series.11
Incidence and scope of the problem
The published incidence of difficult airway management interventions varies substantially (Table 1). Although different definitions, patient populations, and clinician experience make these figures difficult to compare directly, a few trends emerge. Perhaps one of the more significant trends is the higher occurrence of difficulty encountered in locations outside of the operating room (OR).
Management of the difficult and failed airway in the unconscious/induced patient
Most airway management is performed in an unconscious patient, usually pharmacologically induced for surgical anesthesia. Outside the OR environment, a critically ill patient may be induced for the sole purpose of securing the airway or may already have been unconscious on initial presentation.
Airway management in the induced surgical patient may involve SGD or face mask ventilation, tracheal intubation, or rarely, a primary cricothyrotomy or tracheotomy. Difficulty may be encountered with any of these modalities and should be met with an appropriate response.
The primary approach to tracheal intubation: “Plan A”
For the unconscious/induced patient requiring tracheal intubation, the clinician’s primary “Plan A” approach may have been facilitated by DL or an alternative to DL, such as video laryngoscopy. Alternatives to DL may be chosen as the primary approach due to anticipated difficulty with DL, their utility in teaching, or clinician preference. The chosen technique should be suited to the context of patient anatomy and pathophysiology, operator familiarity, and the practice environment. The probability of first-attempt success should be maximized by familiarity with and attention to equipment and adjunct (e.g., malleable stylet or tracheal tube introducer) preparation, patient positioning, and optimal pharmacotherapy.51
Response to difficulty encountered in the unconscious patient
Difficult direct laryngoscopy: If a poor view is obtained during attempted DL despite proper positioning of the patient and the laryngoscope blade tip, optimizing maneuvers should occur, such as application of external laryngeal pressure (Strong recommendation for, level of evidence B). Unless contraindicated by C-spine precautions, additional head lift (to accentuate lower neck flexion and head/upper neck extension) may also be helpful.52-54
External laryngeal pressure is effective at improving the view during DL.55-63 This maneuver is distinct from cricoid pressure, applied to the cricoid cartilage to help prevent passive regurgitation of gastric contents. In studies, cricoid pressure resulted in no improvement64-66 or a worse64,67-69 view with DL; hence, a recommendation can be made against its use for the sole purpose of improving the view during DL if used instead of laryngeal pressure (Weak recommendation against, level of evidence C). External laryngeal pressure and head lift can be performed sequentially during the first attempt at DL.
There is little evidence that an automatic blade change is an effective strategy for a second attempt at DL unless a specific anatomic finding during the initial laryngoscopy suggests a benefit. Examples include a long, floppy epiglottis that could be directly elevated with a longer curved, or straight blade, or a suspicion that a Macintosh blade is too short to completely advance into the vallecula, thus failing to engage the underlying hyoepiglottic ligament.
The tracheal tube introducer has been extensively studied as an adjunct to DL. It is an effective aid to tracheal intubation faced with a restricted view during DL20,25,38,70-74 and may also be useful with some video laryngoscopes. If a restricted (e.g., Cormack-Lehane grade 2b or 3)19,20 view obtained during DL persists after optimization maneuvers such as external laryngeal pressure or additional head lift, use of a tracheal tube introducer should be considered (Strong recommendation for, level of evidence B). The CAFG recommends immediate availability of a tracheal tube introducer at all airway management locations.
Difficult video laryngoscopy: There are three independent tasks with video laryngoscopy, namely, laryngeal exposure, delivery of the tracheal tube to the laryngeal inlet, and advancement within the trachea. Use of a video laryngoscope will generally result in a good laryngeal view, although blades with more angulation or curvature will enable better exposure. The following techniques can facilitate passage of the tracheal tube: preparing a tracheal tube with a preloaded stylet with a curvature matching that of the video laryngoscope blade, partial withdrawal of the blade to provide a wider visual field, and deliberately not seeking a full view of the larynx before attempting passage of the tube. Once placed through the glottic opening, withdrawing the stylet 5 cm will help circumvent impingement of the tracheal tube tip on the anterior tracheal wall, permitting gentle tube advancement. Rotation of the tube may also address impingement. Video laryngoscopes with channeled blades (e.g., Airtraq®, Ambu® AWS, and KingVision™) also exist to facilitate delivery of the tracheal tube. Failure to achieve a view of the larynx with video laryngoscopy can be minimized by suctioning the oropharynx prior to initial blade insertion.
Difficult face mask ventilation: Difficult face mask ventilation of the unconscious patient before or between tracheal intubation attempts should be addressed with a graduated response, including placement of an appropriately sized oropharyngeal and/or nasopharyngeal airway, use of a two-handed mask hold, and exaggerated head extension, unless contraindicated (Strong recommendation for, level of evidence C).
The two-handed face mask hold facilitates ventilation by projecting the mandible anteriorly into the mask, which pulls the tongue forward and further opens the airway. It also provides an improved mask seal. Ventilation can be provided by an assistant or by the anesthesia machine ventilator if the patient is in the OR.
Cricoid pressure may make face mask ventilation difficult, especially if applied with excess force.75 If cricoid pressure has been applied and difficult face mask ventilation is deemed unresponsive to the foregoing measures, progressive release of pressure should be considered (Weak recommendation for, level of evidence C).
If difficult or impossible face mask ventilation persists despite corrective maneuvers, a SGD should be placed or tracheal intubation should be undertaken if not already attempted.15,76,77 Failure to ventilate with a SGD can often be resolved by ensuring an adequate depth of anesthesia, appropriate (e.g., no more than 60 cm H2O) cuff inflation, reinsertion of the device with a fully deflated cuff, or placement of a larger SGD.
Unsuccessful primary approach to tracheal intubation
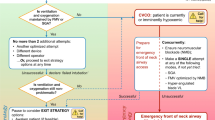
An attempt at tracheal intubation may be unsuccessful despite optimized conditions and technique. In the induced/unconscious patient, this will most often be followed by face mask ventilation or, optionally, placement of a SGD. The success of oxygenation by face mask or SGD ventilation in this context dictates subsequent actions (Fig. 1). As long as oxygenation is non-problematic, the situation is stable, and if deemed appropriate, time exists for additional careful attempts at tracheal intubation. Conversely, the failure of face mask ventilation or a SGD to maintain adequate oxygenation after a failed attempt at tracheal intubation indicates a failed oxygenation/CICO situation (represented in the Emergency pathway on the right-hand side of the Fig. 1 flow diagram).
With non-problematic oxygenation, a second attempt at tracheal intubation can occur using the primary “Plan A” technique, but only if it is reasonable to presume that the factors contributing to the initial unsuccessful attempt can be addressed during the subsequent attempt. For example, an unsuccessful primary attempt at intubation using video laryngoscopy may yield information about the ideal curvature of a tracheal tube with preloaded stylet required for a second attempt.
The alternative approach to tracheal intubation: “Plan B” in the adequately oxygenated patient
An alternative “Plan B” approach to tracheal intubation should be employed if the primary approach is unsuccessful, if oxygenation remains non-problematic, and if further intubation attempts are planned. Experienced providers will often proceed to the alternative approach after only a single failed attempt with the primary device, recognizing the low incremental probability of successful intubation with a second attempt using the same device. In general, the alternative approach should be used after no more than two failed attempts at tracheal intubation using the primary approach and should employ a different device or operator.
Numerous alternatives to DL, used alone or in combination, have been proven effective in obtaining an improved view of the larynx and/or enabling successful tracheal intubation when DL is unsuccessful (Table 2). Many of the devices presented in Table 2 are indirect (e.g., video) laryngoscopes, although other techniques are also effective in experienced hands. Equally, there is also some evidence that DL-facilitated intubation may succeed should primary use of some of these same alternatives fail.78,79 As such, an argument can be made that these alternative devices should complement and not necessarily replace DL at this time. Irrespective of the technique chosen, proficiency demands elective experience in human subjects.
There should be a reasonable expectation that the selected “Plan B” technique will address the reason, anatomic or otherwise, for failure of the primary approach. As with the primary approach, each use of the alternative device should be optimized, and a second attempt using the same device should occur only if made with a substantive change, e.g., a change in the size of the device, altered endotracheal tube/stylet conformation, or use by a more experienced operator. All clinicians with a mandate for airway management should be familiar with at least one alternative technique (e.g., video laryngoscopy) to DL to enable tracheal intubation (Strong recommendation for, level of evidence C), and such equipment should be immediately available. When difficult or failed DL is encountered, proceeding with a “Plan B” alternative intubation technique without awakening the elective surgical patient is common practice and is probably safe, provided that oxygenation remains unchallenged.
Failed tracheal intubation in the adequately oxygenated patient: exit strategies
Limits to tracheal intubation attempts
Evidence continues to emerge that patient morbidity increases with the number of attempts at tracheal intubation (Table 3). Mainly derived from the critically ill population, it must be acknowledged that there is marked heterogeneity in harmful “outcomes” reported in these studies (e.g., aspiration, hypoxemia, hypotension, trauma etc.), including composite outcomes. Furthermore, there is variable use of neuromuscular blockade, and it is unclear if the apparent risk relates to the number of attempts required, additional exerted force, or the associated delay in successful intubation. Nevertheless, the studies do provide a warning that the number of attempts at tracheal intubation should be minimized, irrespective of practice location. Incremental risk must be assumed with each failed attempt such that a second or third tracheal intubation attempt should occur only if a different tactic is used and there is a reasonable expectation of success. Proceeding with more than three attempts at tracheal intubation requires compelling justification.
With the evidence of harm accruing from multiple attempts at tracheal intubation, an argument can be made for always including first-attempt success rates in future studies of intubation devices, techniques, or skills acquisition.
Failed tracheal intubation: exit strategies
Three failed attempts at tracheal intubation should be taken as an indication to declare a failed intubation situation. This should signal the team to pause and consider an exit strategy, to avoid repetitive ineffective intubation attempts that might result in harm to the patient. In the adequately oxygenated unconscious/induced patient, a number of exit strategies exist:
-
Awakening the patient. The option of allowing the induced oxygenated patient to wake after failed tracheal intubation should be considered when feasible (Weak recommendation for, level of evidence C). Once awake and cooperative, awake tracheal intubation can be attempted in the spontaneously breathing patient. Alternatively, an elective surgical case could be deferred or potentially performed under regional or infiltration anesthesia. Oxygenation should be maintained with face mask or a SGD until the patient emerges from general anesthesia. Awakening the patient may not be possible or appropriate in an emergency, during an attempted resuscitation, or if the patient cannot cooperate with awake intubation or surgery under regional anesthesia. While there is no evidence to support the contention that awakening the elective surgical patient will confer an outcome benefit when tracheal intubation has failed, this option is supported by expert consensus to prevent deterioration to a failed oxygenation, “cannot intubate, cannot oxygenate” scenario.
-
Proceeding with surgery (or temporizing an emergency situation) using face mask or SGD ventilation. As an exit strategy for failed tracheal intubation in the induced/unconscious patient, the benefit of proceeding with surgery under face mask or SGD ventilation must exceed the risk of foregoing tracheal intubation. In general, this will be easier to justify for brief or urgent surgeries, although risk of aspiration must be considered. If surgery proceeds under face mask or SGD ventilation, a plan should exist for difficulty with or failure of oxygenation during the case. The critically ill non-surgical patient temporized with face mask or SGD ventilation will likely still require tracheal intubation or a surgical airway, sooner rather than later.
-
Obtaining equipment or additional expert help for a further controlled attempt at tracheal intubation. There is no doubt that minimizing tracheal intubation attempts is a sound principle. Nevertheless, the goal of engaging an exit strategy is not necessarily to prohibit more than three intubation attempts so much as to serve as a warning that further attempts may be attended by increasing patient harm and decreasing chances of success. Consequently, an “exit strategy” attempt at tracheal intubation should occur only with a high likelihood of success and a low probability of creating complications. For example, if a SGD had been placed after three failed attempts at tracheal intubation, bronchoscopy-aided intubation could have ensued via the SGD once an appropriate flexible bronchoscope became available. Alternatively, if additional expert help had been available, another attempt at intubation could have occurred with the same or a different device, being mindful of the need to avoid traumatizing the airway during the attempt.
-
Proceeding with surgical access. In rare circumstances, it may be appropriate to proceed with surgical access (cricothyrotomy or tracheotomy) following failed tracheal intubation in the adequately oxygenated unconscious/induced patient. This may be required if awakening the patient is not an option, i.e., most often in urgent or emergency situations.
Failed tracheal intubation may be apparent and an exit strategy engaged before three attempts at intubation have occurred, even after a single unsuccessful attempt.
Failed oxygenation during attempted tracheal intubation: the emergency strategy
Failed oxygenation (“cannot intubate, cannot oxygenate” [CICO]) exists following failed tracheal intubation if the patient cannot be successfully oxygenated by optimized face mask or SGD ventilation (Fig. 1). Three corrective measures are vital: immediate recognition, a call for help, and preparation for proceeding rapidly with a surgical/transtracheal airway (most often cricothyrotomy in the adult patient).
Due to the rarity of this situation, clinicians commonly exhibit a lack of situation awareness when failed oxygenation/CICO is encountered, having become fixated on multiple unproductive attempts at tracheal intubation or SGD placement. The failure to recognize failed oxygenation/CICO and respond appropriately has been shown to delay cricothyrotomy, resulting in cerebral hypoxia and cardiac arrest.6,9 It is imperative that all members of the assembled team be empowered to call for help or raise the need for emergency cricothyrotomy.
The Focus Group was reluctant to recommend a specific arterial oxygen saturation (SaO2) trigger for cricothyrotomy in a failed oxygenation/CICO situation. Nevertheless, given the sigmoid shape of the oxyhemoglobin dissociation curve, as SaO2 descends through 90%, the rate of desaturation will accelerate if efforts at oxygenation remain unsuccessful. A failed oxygenation/CICO situation with a rapidly declining SaO2 despite maximum attempts at oxygenation should be taken as an indication for cricothyrotomy, especially with the onset of bradycardia.172,173
Published case series174-176 and reports38,177-180 have described successful rescue oxygenation in failed oxygenation/CICO scenarios with placement of a SGD. Although recommended by national guidelines in many countries,12,17,172,181,182 evidence is lacking on whether outcome is improved with attempted SGD placement prior to cricothyrotomy in failed oxygenation/CICO situations. Regardless, if failed oxygenation/CICO occurs, one attempt should be made at placing an appropriately sized SGD familiar to the operator, unless this has previously failed (Strong recommendation for, level of evidence C). During this SGD attempt, a second individual should simultaneously prepare equipment and the patient’s neck for cricothyrotomy. If oxygenation is not restored via the SGD, immediate cricothyrotomy should proceed without further attempts at either SGD placement or transglottic tracheal intubation (Strong recommendation for, level of evidence C). As it takes longer than cricothyrotomy, retrograde intubation is not recommended in failed oxygenation/CICO scenarios.
For emergency subglottic transtracheal access, cricothyrotomy is most often recommended in adults over tracheotomy, particularly when performed by a non-surgeon. This is advocated because the space is less vascular and more readily palpable.
Cricothyrotomy can be categorized as surgical or non-surgical. Surgical cricothyrotomy involves the use of a scalpel to incise the skin and cricothyroid membrane, with placement of a small (e.g., 6.0-mm internal diameter [ID] in the adult) endotracheal or tracheostomy tube. Other instruments needed for the procedure may include a tracheal hook, a Trousseau dilator, or a tracheal tube introducer.183
Non-surgical cricothyrotomy involves one of two options: percutaneous insertion of a wide bore (≥ 4-mm ID) cannula by either cannula-over-needle or Seldinger wire-guided (e.g., Melker) techniques, or percutaneous insertion of a narrow bore (≤ 2-mm) intravenous-type cannula. Narrow-bore cricothyrotomy with jet ventilation requires a high-pressure ventilation source in adults (not universally available in all airway management locations); it is more likely to result in breath stacking, barotrauma, catheter kinking, or dislodgement, and does not provide airway protection with a cuff. Of the available options, it is associated with the highest complication and failure rates.6,9,10 Unless the clinician is very experienced with jet ventilation, this suggests that options in failed oxygenation/CICO in the adult patient should be limited to either the percutaneous needle-guided wide-bore cannula or the open surgical technique (Strong recommendation for, level of evidence C). Both percutaneous wide-bore cannula and open surgical choices allow the desirable option of placing a cuffed tracheal cannula/tube.
There is some evidence that the percutaneous needle-guided wide-bore cannula technique may be less effective than the open surgical procedure.9,10,184 Nevertheless, a recent survey suggested that Canadian anesthesiologists were most comfortable with a percutaneous technique.185 On balance, we recommend that adult cricothyrotomy should proceed with either a percutaneous needle-guided wide-bore cannula or an open surgical technique, governed by operator preference and equipment availability. Even so, mindful of the significant reported failure rates of the percutaneous techniques, clinicians must be prepared for immediate conversion to an open surgical technique should the percutaneous needle-guided technique fail.
Recent studies suggest that anesthesia providers may have difficulty with correctly identifying the cricothyroid membrane using external landmarks.186,187 This may argue for always beginning cricothyrotomy with a 3-cm vertical midline incision over the presumed location of the cricothyroid membrane (Weak recommendation for, level of evidence C), at least in the patient with indistinct external landmarks. The cricothyroid membrane may then be more accurately identified within the incision, and the cricothyrotomy can continue with either a needle-guided wide-bore cannula or surgical technique.
As one of the major complications of cricothyrotomy placement is false passage, correct cannula or tube location must be objectively confirmed using capnography or endoscopy.
Even if administering (or re-dosing) a neuromuscular blocking agent is not indicated as part of the initial management plan, once a failed oxygenation/CICO situation occurs, it should be considered to address possible laryngospasm and facilitate face mask ventilation (Weak recommendation for, level of evidence C).48 Secondly, if bradycardia should occur, administration of epinephrine or atropine may forestall cardiac standstill. In both instances, these actions are to be delegated to an assistant and must not delay cricothyrotomy.
As an infrequently-performed yet life-saving procedure, all airway managers must acquire and maintain cricothyrotomy skills through educational programs. Cricothyrotomy equipment should be readily accessible, and all clinicians and ancillary staff should know its location.
Tracheal intubation confirmation
The persistent presence of exhaled carbon dioxide “appropriate to the clinical circumstance” provides objective confirmation of tracheal intubation.12 Visualization of a tracheal tube between the cords or endoscopic visualization of the subglottic airway through a tracheal tube can provide additional confirmation.12 Chest rise and auscultation, tube misting, chest radiography, and pulse oximetry are not robust indicators of successful tracheal intubation.
In the NAP4 study, many complications of airway management reported in the emergency department (ED) and ICU were related to unrecognized esophageal intubation or tracheal tube dislodgements. The inconsistent use of capnography for confirmation of tracheal intubation or the lack of continuous capnographic monitoring of already intubated patients was judged contributory.10 Thus, capnographic confirmation of tracheal tube placement should occur for all hospitalized patients (Strong recommendation for, level of evidence B), and ongoing continuous waveform capnographic monitoring should occur for the duration of intubation and ventilation (Strong recommendation for, level of evidence C). The latter recommendation will facilitate early detection of tube dislodgement as well as inadvertent hyper- or hypoventilation.
Additionally, NAP4 found that the absence of a capnographic waveform in the setting of cardiac arrest was sometimes incorrectly ascribed to the absence of pulmonary perfusion without consideration of either esophageal intubation or a completely obstructed tracheal tube or trachea.9,10 This occurred in OR, ED, and ICU environments. In actual fact, the first 30 min of cardiac arrest with adequate chest compressions is often associated with an attenuated but present capnography trace when the tracheal tube is correctly situated and unobstructed.188 A flat capnograph should prompt exclusion of a misplaced or blocked tracheal tube.
Continuous capnographic monitoring has also been recommended for patients without tracheal intubation who are undergoing deeper levels of procedural sedation (e.g., Ramsay sedation scores 4-6).189
The obstetric airway: special considerations
A higher incidence of failed tracheal intubation has been reported in the parturient than in the general surgical population.31,32,190 Nevertheless, in series originating in jurisdictions with either a high volume of obstetrical general anesthetics or coverage limited to senior trainee or consultant anesthesia staff, the incidence of failed intubation is more consistent with that of general surgical cases.30,191,192 This should not induce complacency, however, as multiple issues can converge and potentially contribute to airway-related morbidity in the parturient193 (Table 4). To help mitigate these factors, it is essential that obstetrical units have appropriately trained staff and airway equipment that is immediately accessible and of the same quality and type (e.g., video laryngoscopes) as that used in the main surgical ORs of the facility (Strong recommendation for, level of evidence C).
Difficult and failed tracheal intubations may be avoided by the more frequent use of regional anesthesia for obstetric surgical procedures.192,201,202 High levels of anesthetic skill and experience facilitate effective and rapid neuraxial anesthesia in many emergency situations.202 On the other hand, as general anesthesia rates continue to fall, there is ongoing concern that trainees are not being adequately exposed to airway management of the parturient—many tertiary care centres now typically have general anesthesia rates of 5-7% for Cesarean delivery.32,202
Avoiding a bad airway-related outcome – first steps: Antenatal airway screening of all parturients should ideally occur to identify potential challenges.30,203-205 Once a parturient with difficult airway anatomy is identified, good communication is crucial. A plan should be formulated with the attending obstetrician with the understanding that, if operative delivery is likely, it should occur under controlled conditions. Early placement of an epidural catheter should be considered. The catheter should be tested to confirm its efficacy so that rapid conversion to a surgical level of anesthesia can occur for emergency Cesarean delivery. If the epidural is not working and time permits, it should be re-sited. Once the need for general anesthesia becomes apparent, the attending anesthesiologist should perform a formal assessment of the airway. The patient should be given pharmacologic anti-aspiration prophylaxis (Strong recommendation for, level of evidence C).
For induction of general anesthesia, all parturients should be appropriately positioned (e.g., “ramped” as needed to ensure the patient’s external auditory meatus is level with the sternal notch).206 Pre-oxygenation should occur using high flow rates of oxygen, with tidal volume breathing for three minutes, if time permits, or eight deep breaths over 60 sec207 (Strong recommendation for, level of evidence B). Cricoid pressure should be applied with induction and maintained as appropriate until the airway is secured. Succinylcholine is generally used to facilitate laryngoscopy if no contraindication exists. After induction, face mask ventilation with low insufflation pressures can occur while awaiting full onset of neuromuscular blockade. This is carried out both to extend oxygenated apnea time during tracheal intubation and to anticipate ease of face mask ventilation should a first attempt at intubation fail (Strong recommendation for, level of evidence C). Although this recommendation is a departure from the classic teaching of avoiding face mask ventilation during rapid sequence induction, the potential benefit of oxygenation probably outweighs the small risk of gastric insufflation causing regurgitation, especially if insufflation pressures are kept < 20 cm H2O.172,208
Failed primary attempt at intubation encountered in an induced/unconscious parturient: If a first attempt at tracheal intubation fails despite optimized technique, gentle face mask ventilation should be resumed (Fig. 2), and help summoned. Cricoid pressure should be maintained unless thought to be contributing to difficulty. Any difficulty with face mask ventilation should be met with a standard response of oropharyngeal airway insertion, two-handed mask hold with exaggerated jaw thrust, incremental release of cricoid pressure, and if necessary, SGD placement. If oxygenation is non-problematic, a second tracheal intubation attempt can occur with the following provisos: there must be a reasonable likelihood of success based on findings at the initial attempt and a different technique (e.g., video laryngoscopy) or operator should be employed.
Exit strategy – failed tracheal intubation in the oxygenated parturient with NO fetal or maternal emergency: If tracheal intubation has failed and further attempts are predicted to have a low incremental likelihood of succeeding, the acuteness of the situation should be assessed. With no fetal or maternal emergency, the goal should be to maintain oxygenation and allow the parturient to emerge from general anesthesia. At that point, a decision can be made to revisit regional anesthesia (if not contraindicated) or proceed with awake tracheal intubation for general anesthesia. If face mask ventilation becomes difficult, a SGD should be placed to assist oxygenation while awaiting emergence from anesthesia. Use of a SGD with a second lumen to allow esophageal and gastric venting should be considered.
Exit strategy – failed tracheal intubation in the oxygenated parturient WITH fetal or maternal emergency: If persistent fetal distress or a maternal emergency exists following failed tracheal intubation in the adequately oxygenated parturient, Cesarean delivery and/or maternal resuscitation can proceed with face mask or SGD ventilation. Cricoid pressure should be released for SGD insertion. Most Focus Group members agree that re-applying cricoid pressure is unlikely to be beneficial after placement of a SGD with an esophageal port. After failed tracheal intubation for Cesarean delivery under face mask or SGD ventilation in an emergency, the obstetrician should be requested to make a generous surgical incision and to minimize fundal pressure or use vacuum extraction at the time of delivery209 (Strong recommendation for, level of evidence B). With uncomplicated and expeditious surgery, the procedure can be completed with face mask or SGD ventilation. If the case is complex, once the fetus has been delivered or the maternal emergency is stabilized, a cuffed tracheal tube can be placed under more controlled conditions (e.g., flexible bronchoscopic-aided intubation through a SGD), if required. If conditions permit, the surgery should be halted temporarily while the airway is secured, with optimized patient positioning and obstructing drapes moved aside.
A number of observational studies from outside North America have been published on using SGDs for elective Cesarean delivery in a select group of women. The subjects in these studies were of normal body mass index and well-fasted; they had anti-aspiration prophylaxis and underwent quick uncomplicated surgery. Although each study used a different version of the Laryngeal Mask Airway (LMA™), they were consistent in reporting a high rate of successful SGD placement and ventilation.44-46 In North America, with general anesthesia reserved mainly for emergency cases and with parturients typically having a higher body mass index, SGDs cannot be recommended for elective Cesarean delivery at this time (Strong recommendation against, level of evidence B). Nevertheless, these and other studies190 do support the early use of a SGD in any airway rescue scenario in the parturient (Strong recommendation for, level of evidence B).
Emergency strategy – failed intubation, oxygenation NOT possible with face mask or SGD ventilation: Following a failed attempt at tracheal intubation, the failure to oxygenate the parturient with face mask or SGD ventilation (failed oxygenation/CICO) will also quickly result in fetal compromise. As with the general surgical patient, the default response to this scenario is cricothyrotomy, with a parallel bridging attempt at oxygenation with a SGD if not already tried. Once the patient is re-oxygenated via SGD or cuffed cricothyrotomy cannula, Cesarean delivery or further resuscitation can occur if a fetal or maternal emergency exists; however, if the situation is now stable, optionally, the patient can be awakened and a plan can be made for definitive care.
It must be emphasized that the failed oxygenation/CICO scenario implies a complete inability to oxygenate the patient. In this situation, the parturient will undergo rapid oxygen desaturation, indicating why further attempts at tracheal intubation are contraindicated and also why it would be impractical to allow the mother to wake.
Extubation and the postpartum period: Recent maternal mortality statistics from both the United States and United Kingdom indicate a shift in many airway catastrophes from induction of general anesthesia to the postpartum period, i.e., at emergence, in the postanesthesia unit, or when applied for postpartum surgical procedures.210,211 Heightened vigilance during these phases is clearly required.
The pediatric airway: special considerations
Respiratory complications continue to be a major source of morbidity in children requiring airway management.212,213 Despite this, difficult DL is rare in an otherwise healthy child. In an audit of 11,219 pediatric general anesthetics in a tertiary care centre, the incidence of difficult DL (Cormack-Lehane grade 3 or 4 views) was 4.7% in children less than one year of age and 0.7% in children older than one year.214 In another audit of 24,165 anesthetics in a tertiary care pediatric centre, the frequency of unanticipated difficult tracheal intubations was 0.24% in children less than one year of age and 0.07% in children older than one year.213 These figures may reflect a higher than expected incidence compared with that encountered in community hospitals due to referral bias.
Unexpected difficult face mask ventilation is also rare in pediatrics. When difficult mask ventilation is encountered, causes such as laryngospasm or gastric distension must be considered. Clinicians should include the unexpected in their differential diagnosis, such as congenital airway anomalies or airway obstruction by foreign bodies.215 The pediatric airway is very susceptible to trauma when compared with the adult airway, and repeated attempts at intubation may result in more swelling and subsequent airway compromise. Rapid desaturation during apnea and a lack of patient cooperation are additional significant considerations.
Video laryngoscopy: Many case reports describe video laryngoscopy facilitating successful tracheal intubation in children with difficult airways. As with adults, the majority of current studies show that use of certain video laryngoscopes can facilitate an improved glottic view when compared with DL in pediatric patients with a reassuring airway exam. However, time to intubation is either unchanged or prolonged.216-219 In one pilot study in pediatric patients with known or anticipated difficult airways, use of the GlideScope Cobalt™ resulted in a significantly improved glottic view compared with DL in 17 of 18 patients, although tracheal intubation failed when using the device in three of the 18 patients.220 Despite the lack of published pediatric studies, video laryngoscopy has the potential to be useful in the pediatric difficult airway.
Cuffed vs uncuffed tracheal tubes in children: There is no direct evidence that use of a cuffed tracheal tube in children will cause more subglottic injury or iatrogenic stenosis than an uncuffed tube.221,222 Use of a cuffed tracheal tube will minimize need for re-intubation,221 decrease the potential for loss of effective ventilation,223 and may protect against micro-aspiration.224 As long as close attention is paid to maintaining an adequate air leak (i.e., occurring at < 20-25 cm H2O) and/or monitoring cuff pressure, a recommendation can be made to use cuffed tracheal tubes for all difficult or emergency pediatric tracheal intubations (Strong recommendation for, level of evidence B).
SGDs in the difficult pediatric airway: Apart from case reports, little published evidence exists on the use of SGDs in the setting of difficult DL, difficult airway, or failed oxygenation/CICO situations in children. Case series support the use of SGDs, such as the LMA Classic™ and the air-Q® Intubating Laryngeal Airway, as conduits for intubation when difficult pediatric DL is encountered or anticipated.99,100,225-227 In most of these series, intubation was facilitated with flexible or semi-rigid endoscopy through the SGD. In a failed oxygenation/CICO situation, as with adult recommendations, an attempt should be made to oxygenate the pediatric patient with a SGD while equipment is being prepared for a surgical airway.
Transtracheal/surgical airway: Failed oxygenation/CICO situations are rare in children. The best strategy for emergency transtracheal oxygenation in children under 8-10 years of age remains unclear. In this population, the cricothyroid space is underdeveloped, leaving needle tracheotomy or surgical tracheotomy below the cricoid ring as the only options for transtracheal access. Depending on the pathology (e.g. subglottic stenosis, tracheal foreign body), rigid bronchoscopy may be the intervention of choice. In children older than eight to ten years of age, the vertical span of the cricothyroid space enlarges sufficiently to accommodate several of the commercially available cricothyrotomy products, although some of these devices have been associated with tracheal damage in animal models.228,229
The few reports on emergency transtracheal airway access in children under age 18 vary greatly in circumstances, equipment used, and patient age.9,230-233 Experience with transtracheal catheters placed for elective pediatric surgical procedures suggests that, despite controlled conditions, use of jet ventilation through such catheters is associated with a significant rate of complications, including barotrauma.234-236 Animal237,238 and bench239 modelling indicate that adequate oxygenation can be provided through transtracheal catheters without the use of jet ventilation.
In children younger than eight to ten years in a failed oxygenation/CICO situation, help should be summoned, and if not already attempted, a SGD should be placed while equipment is readied for surgical or needle tracheotomy (or rigid bronchoscopy, when indicated) (Strong recommendation for, level of evidence C). For the needle tracheotomy option, a kink-resistant240 catheter specifically made for this purpose should be used. Oxygenation can be provided via an Enk Oxygen Flow Modulator™ (Cook Medical, Bloomington, IN) with a flow rate of 1 L per year of age239 and an inspiratory-to-expiratory (I:E) ratio sufficient to allow expiration. As full expiration of tidal volume will not occur through the transtracheal catheter, continued attempts at airway-opening maneuvers and securing a definitive airway are essential.
Documentation following an encounter with a difficult airway
Appropriate documentation should be completed following every airway intervention, difficult or otherwise. The record should make specific mention of ease of face mask or SGD ventilation, the device used to perform tracheal intubation, the view obtained, and the number of attempts (Strong recommendation for, level of evidence C).
If airway management is difficult once, it seems intuitive that subsequent attempts will also be difficult, although patient, operator, or equipment factors may differ significantly. There is some evidence that a previously designated difficult or failed DL or intubation does confer a higher likelihood of encountering similar circumstances on a subsequent occasion.29,241,242 However, pertinent high-level prospective outcome studies using precise definitions are currently lacking, and may never be published. Even so, experts agree that it seems likely that good documentation and dissemination of difficult airway information may reduce critical airway events. The CAFG advocates a multi-layered strategy appropriate to the local system when a difficult airway situation has been encountered. At a minimum, this should include clear and accurate documentation in the patient’s medical record, personally informing the patient and the patient’s surgeon, and providing a difficult airway letter to the patient with copies to both the chart and the primary care provider.
Electronic recording and alert systems are advances over traditional handwritten records. In-hospital alert bracelets and local or national databases (e.g., the MedicAlert Foundation) should also be considered. Such databases have the advantage of being widely accessible without restriction of space or jurisdiction.
While subjective, the trigger for invoking this multi-layered strategy may include factors such as an inability to visualize the larynx, very difficult or impossible face mask ventilation, or opinion that future airway interventions would occur most safely with the patient awake.
Copies of a difficult airway alert letter (e.g., Appendix 1) should be stocked in locations where airway management regularly occurs. The content and structure of information contained in airway alerts should be clear and complete to maximize both patient safety and the potential for future database research.
The corollary of performing good documentation is the need for clinicians to augment the bedside airway assessment by seeking additional information from a hospital chart, letter, or database sources, when available, especially when significant difficulty is anticipated. Nevertheless, as highlighted by NAP4, anticipating difficulty is of no benefit unless the airway management strategy is modified accordingly.243
Education in difficult airway management
Management of the difficult airway requires technical and non-technical skills.244 Technical skills are defined as the specific medical knowledge and procedural ability required for managing the airway. Non-technical skills are generalizable skills required to manage dynamic high-risk/low-frequency crisis situations. These non-technical skills include leadership, teamwork, situational awareness, task management, and decision-making.245
Dedicated experiential learning and deliberate practice is beneficial for airway management, but because difficult airways are low-frequency events, it is not appropriate to learn best management algorithms and techniques in the clinical setting.246 As an alternative, simulation provides a proven platform for the acquisition of airway-related technical skills without risk to patients. These skills transfer well to the clinical setting across different learner experience and various device and simulation modalities.247-250 Unfortunately, learning patterns and curves of airway-related technical skills cannot be generalized, as they vary and depend on a clinician’s cumulative experience in the simulated and live setting.251-254 There is no “magic number” for competence in using a particular device or for managing a specific situation. Non-technical skills must also be learned and have been shown to improve with repeated simulation scenarios;255-257 however, further research is needed to show that the acquisition of non-technical skills translates to improved patient outcomes in the clinical setting.
Most importantly, there is demonstrable evidence that both technical and non-technical skills in difficult airway management weaken with time.258,259 The infrequency of these clinical events demands that proficiency be addressed through continuing education workshops that provide an opportunity for active experiential learning and formative assessment with feedback. Simulation has been used to improve difficult airway management skills in practicing physicians, with improvement being retained for as long as a year.260 Educators are currently researching the maximum time interval before significant attrition of skills in order to guide continuing professional development revalidation guidelines.
Summary of recommendations
Face mask ventilation
-
1.
Difficult mask ventilation of the unconscious patient should be met with a graduated response, including use of an oropharyngeal and/or nasopharyngeal airway, use of a two-handed face mask hold, and exaggerated head extension, unless contraindicated – Strong recommendation for, level of evidence C.
-
2.
If difficult face mask ventilation is encountered unresponsive to standard measures of oropharyngeal airway insertion, two-handed mask hold and exaggerated head extension, a trial of progressive release of any applied cricoid pressure should be considered – Weak recommendation for , level of evidence C.
Supraglottic device use
-
1.
If a failed oxygenation, “cannot intubate, cannot oxygenate” (CICO) situation occurs, one attempt at placing an appropriately sized SGD familiar to the operator should be performed to attempt rescue oxygenation, unless this has previously failed – Strong recommendation for, level of evidence C.
Tracheal intubation
-
1.
All clinicians with a mandate for airway management should be familiar with at least one alternative technique to DL (e.g., video laryngoscopy) to enable tracheal intubation – Strong recommendation for , level of evidence C.
-
2.
If a poor view is obtained during DL despite an appropriately positioned patient and laryngoscope blade tip, external laryngeal pressure should be applied to improve the view – Strong recommendation for , level of evidence A.
-
3.
Cricoid pressure should not be applied for the sole purpose of improving the view during DL – Weak recommendation against , level of evidence B.
-
4.
If a restricted view obtained during DL persists after optimization maneuvers such as application of external laryngeal pressure or additional head lift, use of a tracheal tube introducer should be considered – Strong recommendation for, level of evidence B.
-
5.
Capnographic confirmation of tracheal tube placement should occur for all patients in all hospital locations – Strong recommendation for, level of evidence B.
-
6.
Continuous capnographic monitoring should occur in all hospital locations for all patients with an intubated trachea – Strong recommendation for, level of evidence C.
-
7.
If failed intubation is encountered, when feasible, the option of allowing an induced oxygenated patient to wake should be considered as an exit strategy – Weak recommendation for , level of evidence C.
Emergency surgical airway
-
1.
In a failed oxygenation/CICO situation, if oxygenation is not restored via a SGD, immediate cricothyrotomy should occur without further attempts at either SGD placement or transglottic tracheal intubation – Strong recommendation for, level of evidence C.
-
2.
For emergency cricothyrotomy in the adult patient, unless the clinician is very experienced with jet ventilation, options should be limited to either the percutaneous needle-guided wide-bore cannula or an open surgical technique – Strong recommendation for , level of evidence C.
-
3.
At least in the patient with indistinct external landmarks in the neck, cricothyrotomy (by any technique) should begin with a 3-cm vertical midline incision over the presumed location of the cricothyroid membrane – Weak recommendation for , level of evidence C.
-
4.
Even if not indicated as part of the initial management plan, once a patient is in a failed oxygenation/CICO situation, administering (or re-dosing) a neuromuscular blocking agent should be considered to address possible laryngospasm and facilitate face mask ventilation – Weak recommendation for , level of evidence C.
Obstetrics
-
1.
After failed tracheal intubation during induction of GA for emergency Cesarean delivery, if proceeding under face mask or SGD ventilation, the obstetrician should be requested to make a generous surgical incision and to minimize fundal pressure or use vacuum extraction at the time of delivery – Strong recommendation for, level of evidence B.
-
2.
Early use of a SGD should be considered in any airway rescue scenario in the parturient – Strong recommendation for, level of evidence B.
-
3.
As with the general surgical patient, the default response to a failed oxygenation/CICO scenario in a parturient is cricothyrotomy, with a parallel bridging attempt at oxygenation with a SGD if not already tried – Strong recommendation for, level of evidence B.
-
4.
Obstetrical units should have appropriately trained staff and good, easily accessible airway equipment of the same quality and type (e.g., video laryngoscopy) as that used in the main surgical ORs of the facility – Strong recommendation for, level of evidence C.
-
5.
Once the need for general anesthesia becomes apparent, the attending anesthesiologist should perform a formal airway assessment of the obstetrical patient, including localization of the cricothyroid membrane – Strong recommendation for, level of evidence C.
-
6.
For induction of general anesthesia in the parturient, appropriate patient positioning and pre-oxygenation should occur – Strong recommendation for, level of evidence C.
-
7.
With induction of general anesthesia in the parturient, face mask ventilation with low insufflation pressures can occur after induction while awaiting onset of the full effect of a neuromuscular block – Strong recommendation for, level of evidence C.
Pediatrics
-
1.
Cuffed endotracheal tubes should be used in difficult or emergency pediatric tracheal intubation – Strong recommendation for, level of evidence B.
-
2.
For children younger than 8-10 years in a failed oxygenation/CICO situation, help should be summoned, and if not already attempted, a SGD should be placed while equipment is readied for surgical or needle tracheotomy (or rigid bronchoscopy, when indicated) – Strong recommendation for , level of evidence C.
Documentation
-
1.
Appropriate documentation should be completed following every airway intervention, difficult or otherwise. The record should make specific mention of the ease of face mask or SGD ventilation, the device used to perform tracheal intubation, the view obtained, and the number of attempts – Strong recommendation for, level of evidence C.
References
Sakles JC, Chiu S, Mosier J, Walker C, Stolz U. The importance of first pass success when performing orotracheal intubation in the emergency department. Acad Emerg Med 2013; 20: 71-8.
Hasegawa K, Shigemitsu K, Hagiwara Y, et al. Association between repeated intubation attempts and adverse events in emergency departments: an analysis of a multicenter prospective observational study. Ann Emerg Med 2012; 60: 749-54.
Martin LD, Mhyre JM, Shanks AM, Tremper KK, Kheterpal S. 3,423 emergency tracheal intubations at a university hospital: airway outcomes and complications. Anesthesiology 2011; 114: 42-8.
Griesdale DE, Bosma TL, Kurth T, Isac G, Chittock DR. Complications of endotracheal intubation in the critically ill. Intensive Care Med 2008; 34: 1835-42.
Mort TC. Emergency tracheal intubation: complications associated with repeated laryngoscopic attempts. Anesth Analg 2004; 99: 607-13.
Peterson GN, Domino KB, Caplan RA, Posner KL, Lee LA, Cheney FW. Management of the difficult airway: a closed claims analysis. Anesthesiology 2005; 103: 33-9.
Cook TM, Scott S, Mihai R. Litigation related to airway and respiratory complications of anaesthesia: an analysis of claims against the NHS in England 1995-2007. Anaesthesia 2010; 65: 556-63.
The Canadian Medical Protective Association. Anesthesia Airway Management. An analysis of the CMPA’s closed legal actions 1993-2003. Ottawa: CMPA; Revised May 2008. Available from URL: http://www.cmpa-acpm.ca/cmpapd04/docs/resource_files/risk_id/2005/com_ri0507-e.cfm (accessed May 2013).
Cook TM, Woodall N, Frerk C, Fourth National Audit Project. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1: anaesthesia. Br J Anaesth 2011; 106: 617-31.
Cook TM, Woodall N, Harper J, Benger J, Fourth National Audit Project. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 2: intensive care and emergency departments. Br J Anaesth 2011; 106: 632-42.
Law JA, Broemling N, Cooper RM, et al.; for the Canadian Airway Focus Group. The difficult airway with recommendations for management – Part 2 – The anticipated difficult airway. Can J Anesth 2013; 60: this issue. DOI:10.1007/s12630-013-0020-x.
Crosby ET, Cooper RM, Douglas MJ, et al. The unanticipated difficult airway with recommendations for management. Can J Anaesth 1998; 45: 757-76.
Guyatt G, Gutterman D, Baumann MH, et al. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an American College of Chest Physicians Task Force. Chest 2006; 129: 174-81.
Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336: 924-6.
Kheterpal S, Martin L, Shanks AM, Tremper KK. Prediction and outcomes of impossible mask ventilation: a review of 50,000 anesthetics. Anesthesiology 2009; 110: 891-7.
Langeron O, Masso E, Huraux C, et al. Prediction of difficult mask ventilation. Anesthesiology 2000; 92: 1229-36.
Apfelbaum JL, Hagberg CA, Caplan RA, et al. Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology 2013; 118: 251-70.
Kheterpal S, Han R, Tremper KK, et al. Incidence and predictors of difficult and impossible mask ventilation. Anesthesiology 2006; 105: 885-91.
Cormack RS, Lehane J. Difficult tracheal intubation in obstetrics. Anaesthesia 1984; 39: 1105-11.
Cook TM. A new practical classification of laryngeal view. Anaesthesia 2000; 55: 274-9.
Yentis SM, Lee DJ. Evaluation of an improved scoring system for the grading of direct laryngoscopy. Anaesthesia 1998; 53: 1041-4.
Adnet F, Racine SX, Borron SW, et al. A survey of tracheal intubation difficulty in the operating room: a prospective observational study. Acta Anaesthesiol Scand 2001; 45: 327-32.
Hung O, Law JA. Advances in airway management. Can J Anesth 2006; 53: 628-31.
Walls RM. The emergency airway algorithms. In: Walls RM, Murphy MF, Luten RC, Schneider RE, editors. Manual of Emergency Airway Management. Philadelphia: Lippincott Williams & Wilkins; 2004. p. 8-21.
Amathieu R, Combes X, Abdi W, et al. An algorithm for difficult airway management, modified for modern optical devices (Airtraq laryngoscope; LMA CTrach™): a 2-year prospective validation in patients for elective abdominal, gynecologic, and thyroid surgery. Anesthesiology 2011; 114: 25-33.
Asai T, Koga K, Vaughan RS. Respiratory complications associated with tracheal intubation and extubation. Br J Anaesth 1998; 80: 767-75.
Yildiz TS, Solak M, Toker K. The incidence and risk factors of difficult mask ventilation. J Anesth 2005; 19: 7-11.
Cattano D, Panicucci E, Paolicchi A, et al. Risk factors assessment of the difficult airway: an Italian survey of 1956 patients. Anesth Analg 2004; 99: 1774-9.
el-Ganzouri AR, McCarthy RJ, Tuman KJ, Tanck EN, Ivankovich AD. Preoperative airway assessment: predictive value of a multivariate risk index. Anesth Analg 1996; 82: 1197-204.
Rocke DA, Murray WB, Rout CC, Gouws E. Relative risk analysis of factors associated with difficult intubation in obstetric anesthesia. Anesthesiology 1992; 77: 67-73.
McDonnell NJ, Paech MJ, Clavisi OM, Scott KL, ANZCA Trials Group. Difficult and failed intubation in obstetric anaesthesia: an observational study of airway management and complications associated with general anaesthesia for caesarean section. Int J Obstet Anesth 2008; 17: 292-7.
Tao W, Edwards JT, Tu F, Xie Y, Sharma SK. Incidence of unanticipated difficult airway in obstetric patients in a teaching institution. J Anesth 2012; 26: 339-45.
Graham CA, Oglesby AJ, Beard D, McKeown DW. Laryngoscopic views during rapid sequence intubation in the emergency department. CJEM 2004; 6: 416-20.
Schwartz DE, Matthay MA, Cohen NH. Death and other complications of emergency airway management in critically ill adults. A prospective investigation of 297 tracheal intubations. Anesthesiology 1995; 82: 367-76.
Benedetto WJ, Hess DR, Gettings E, et al. Urgent tracheal intubation in general hospital units: an observational study. J Clin Anesth 2007; 19: 20-4.
Sakles JC, Laurin EG, Rantapaa AA, Panacek EA. Airway management in the emergency department: a one-year study of 610 tracheal intubations. Ann Emerg Med 1998; 31: 325-32.
Tayal VS, Riggs RW, Marx JA, Tomaszewski CA, Schneider RE. Rapid-sequence intubation at an emergency medicine residency: success rate and adverse events during a two-year period. Acad Emerg Med 1999; 6: 31-7.
Combes X, Le Roux B, Suen P, et al. Unanticipated difficult airway in anesthetized patients: prospective validation of a management algorithm. Anesthesiology 2004; 100: 1146-50.
Rose DK, Cohen MM. The airway: problems and predictions in 18,500 patients. Can J Anaesth 1994; 41(5 Pt 1): 372-83.
Rose DK, Cohen MM. The incidence of airway problems depends on the definition used. Can J Anaesth 1996; 43: 30-4.
Ramachandran SK, Mathis MR, Tremper KK, Shanks AM, Kheterpal S. Predictors and clinical outcomes from failed Laryngeal Mask Airway Unique™: a study of 15,795 patients. Anesthesiology 2012; 116: 1217-26.
Baskett PJ, Parr MJ, Nolan JP. The intubating laryngeal mask. Results of a multicentre trial with experience of 500 cases. Anaesthesia 1998; 53: 1174-9.
Ferson DZ, Rosenblatt WH, Johansen MJ, Osborn I, Ovassapian A. Use of the intubating LMA-Fastrach in 254 patients with difficult-to-manage airways. Anesthesiology 2001; 95: 1175-81.
Han TH, Brimacombe J, Lee EJ, Yang HS. The laryngeal mask airway is effective (and probably safe) in selected healthy parturients for elective cesarean section: a prospective study of 1067 cases. Can J Anesth 2001; 48: 1117-21.
Yao WY, Li SY, Sng BL, Lim Y, Sia AT. The LMA Supreme in 700 parturients undergoing cesarean delivery: an observational study. Can J Anesth 2012; 59: 648-54.
Halaseh BK, Sukkar ZF, Hassan LH, Sia AT, Bushnag WA, Adarbeh H. The use of ProSeal laryngeal mask airway in caesarean section—experience in 3000 cases. Anaesth Intensive Care 2010; 38: 1023-8.
Walls RM, Brown CA 3rd, Bair AE, Pallin DJ, NEAR II Investigators. Emergency airway management: a multi-center report of 8937 emergency department intubations. J Emerg Med 2011; 41: 347-54.
Frerk C, Cook T. Management of the ‘can’t intubate can’t ventilate’ situation and the emergency surgical airway. In: Cook T, Woodall N, Frerk C, editors. 4th National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society Major complications of airway management in the United Kingdom. London: The Royal College of Anaesthetists; 2011. p. 105-13.
Dillon JK, Christensen B, Fairbanks T, Jurkovich G, Moe KS. The emergent surgical airway: cricothyrotomy vs. tracheotomy. Int J Oral Maxillofac Surg 2013; 42: 204-8.
Nagaro T, Yorozuya T, Sotani M, et al. Survey of patients whose lungs could not be ventilated and whose trachea could not be intubated in university hospitals in Japan. J Anesth 2003; 17: 232-40.
Benumof JL. Difficult laryngoscopy: obtaining the best view. Can J Anaesth 1994; 41(5 Pt 1): 361-5.
Levitan RM, Mechem CC, Ochroch EA, Shofer FS, Hollander JE. Head-elevated laryngoscopy position: improving laryngeal exposure during laryngoscopy by increasing head elevation. Ann Emerg Med 2003; 41: 322-30.
Schmitt HJ, Mang H. Head and neck elevation beyond the sniffing position improves laryngeal view in cases of difficult direct laryngoscopy. J Clin Anesth 2002; 14: 335-8.
Hochman II, Zeitels SM, Heaton JT. Analysis of the forces and position required for direct laryngoscopic exposure of the anterior vocal folds. Ann Otol Rhinol Laryngol 1999; 108: 715-24.
Benumof JL, Cooper SD. Quantitative improvement in laryngoscopic view by optimal external laryngeal manipulation. J Clin Anesth 1996; 8: 136-40.
Byhahn C, Iber T, Zacharowski K, et al. Tracheal intubation using the mobile C-MAC video laryngoscope or direct laryngoscopy for patients with a simulated difficult airway. Minerva Anestesiol 2010; 76: 577-83.
Latto IP, Stacey M, Mecklenburgh J, Vaughan RS. Survey of the use of the gum elastic bougie in clinical practice. Anaesthesia 2002; 57: 379-84.
Levitan RM, Mickler T, Hollander JE. Bimanual laryngoscopy: a videographic study of external laryngeal manipulation by novice intubators. Ann Emerg Med 2002; 40: 30-7.
Ochroch EA, Levitan RM. A videographic analysis of laryngeal exposure comparing the articulating laryngoscope and external laryngeal manipulation. Anesth Analg 2001; 92: 267-70.
Schmitt H, Buchfelder M, Radespiel-Troger M, Fahlbusch R. Difficult intubation in acromegalic patients: incidence and predictability. Anesthesiology 2000; 93: 110-4.
Takahata O, Kubota M, Mamiya K, et al. The efficacy of the “BURP” maneuver during a difficult laryngoscopy. Anesth Analg 1997; 84: 419-21.
Wilson ME, Spiegelhalter D, Robertson JA, Lesser P. Predicting difficult intubation. Br J Anaesth 1988; 61: 211-6.
Yamamoto K, Tsubokawa T, Ohmura S, Itoh H, Kobayashi T. Left-molar approach improves the laryngeal view in patients with difficult laryngoscopy. Anesthesiology 2000; 92: 70-4.
Noguchi T, Koga K, Shiga Y, Shigematsu A. The gum elastic bougie eases tracheal intubation while applying cricoid pressure compared to a stylet. Can J Anesth 2003; 50: 712-7.
Turgeon AF, Nicole PC, Trepanier CA, Marcoux S, Lessard MR. Cricoid pressure does not increase the rate of failed intubation by direct laryngoscopy in adults. Anesthesiology 2005; 102: 315-9.
Harris T, Ellis DY, Foster L, Lockey D. Cricoid pressure and laryngeal manipulation in 402 pre-hospital emergency anaesthetics: essential safety measure or a hindrance to rapid safe intubation? Resuscitation 2010; 81: 810-6.
Haslam N, Parker L, Duggan JE. Effect of cricoid pressure on the view at laryngoscopy. Anaesthesia 2005; 60: 41-7.
Snider DD, Clarke D, Finucane BT. The “BURP” maneuver worsens the glottic view when applied in combination with cricoid pressure. Can J Anesth 2005; 52: 100-4.
Levitan RM, Kinkle WC, Levin WJ, Everett WW. Laryngeal view during laryngoscopy: a randomized trial comparing cricoid pressure, backward-upward-rightward pressure, and bimanual laryngoscopy. Ann Emerg Med 2006; 47: 548-55.
Combes X, Jabre P, Margenet A, et al. Unanticipated difficult airway management in the prehospital emergency setting: prospective validation of an algorithm. Anesthesiology 2011; 114: 105-10.
Gataure PS, Vaughan RS, Latto IP. Simulated difficult intubation. Comparison of the gum elastic bougie and the stylet. Anaesthesia 1996; 51: 935-8.
Jabre P, Combes X, Leroux B, et al. Use of gum elastic bougie for prehospital difficult intubation. Am J Emerg Med 2005; 23: 552-5.
Nolan JP, Wilson ME. Orotracheal intubation in patients with potential cervical spine injuries. An indication for the gum elastic bougie. Anaesthesia 1993; 48: 630-3.
Greenland KB, Liu G, Tan H, Edwards M, Irwin MG. Comparison of the Levitan FPS Scope™ and the single-use bougie for simulated difficult intubation in anaesthetised patients. Anaesthesia 2007; 62: 509-15.
Hartsilver EL, Vanner RG. Airway obstruction with cricoid pressure. Anaesthesia 2000; 55: 208-11.
Calder I. Impossible mask ventilation. Anesthesiology 2007; 107: 171; author reply 171.
Calder I, Yentis S, Patel A. Muscle relaxants and airway management. Anesthesiology 2009; 111: 216-7; author reply 218-9.
Aziz MF, Healy D, Kheterpal S, Fu RF, Dillman D, Brambrink AM. Routine clinical practice effectiveness of the Glidescope in difficult airway management: an analysis of 2,004 Glidescope intubations, complications, and failures from two institutions. Anesthesiology 2011; 114: 34-41.
Ye L, Wong DT, Liu J, Zhu T. Mallampati class does not affect the success rate of intubation through an intubating laryngeal mask airway with reverse tracheal tube direction. Minerva Anestesiol 2013; 79: 227-31.
Tentillier E, Heydenreich C, Cros AM, Schmitt V, Dindart JM, Thicoipe M. Use of the intubating laryngeal mask airway in emergency pre-hospital difficult intubation. Resuscitation 2008; 77: 30-4.
Dimitriou V, Voyagis GS, Grosomanidis V, Brimacombe J. Feasibility of flexible lightwand-guided tracheal intubation with the intubating laryngeal mask during out-of-hospital cardiopulmonary resuscitation by an emergency physician. Eur J Anaesthesiol 2006; 23: 76-9.
Timmermann A, Russo SG, Rosenblatt WH, et al. Intubating laryngeal mask airway for difficult out-of-hospital airway management: a prospective evaluation. Br J Anaesth 2007; 99: 286-91.
Connelly NR, Ghandour K, Robbins L, Dunn S, Gibson C. Management of unexpected difficult airway at a teaching institution over a 7-year period. J Clin Anesth 2006; 18: 198-204.
Thienthong S, Horatanarung D, Wongswadiwat M, Boonmak P, Chinachoti T, Simajareuk S. An experience with intubating laryngeal mask airway for difficult airway management: report on 38 cases. J Med Assoc Thai 2004; 87: 1234-8.
Cros AM, Maigrot F, Esteben D. Fastrach laryngeal mask and difficult intubation (French). Ann Fr Anesth Reanim 1999; 18: 1041-6.
Joo HS, Kapoor S, Rose DK, Naik VN. The intubating laryngeal mask airway after induction of general anesthesia versus awake fiberoptic intubation in patients with difficult airways. Anesth Analg 2001; 92: 1342-6.
Langeron O, Semjen F, Bourgain JL, Marsac A, Cros AM. Comparison of the intubating laryngeal mask airway with the fiberoptic intubation in anticipated difficult airway management. Anesthesiology 2001; 94: 968-72.
Bein B, Worthmann F, Scholz J, et al. A comparison of the intubating laryngeal mask airway and the Bonfils intubation fibrescope in patients with predicted difficult airways. Anaesthesia 2004; 59: 668-74.
Nakazawa K, Tanaka N, Ishikawa S, et al. Using the intubating laryngeal mask airway (LMA-Fastrach™) for blind endotracheal intubation in patients undergoing cervical spine operation. Anesth Analg 1999; 89: 1319-21.
Fukutome T, Amaha K, Nakazawa K, Kawamura T, Noguchi H. Tracheal intubation through the intubating laryngeal mask airway (LMA-Fastrach) in patients with difficult airways. Anaesth Intensive Care 1998; 26: 387-91.
Brain AI, Verghese C, Addy EV, Kapila A, Brimacombe J. The intubating laryngeal mask. II: a preliminary clinical report of a new means of intubating the trachea. Br J Anaesth 1997; 79: 704-9.
Erlacher W, Tiefenbrunner H, Kastenbauer T, Schwarz S, Fitzgerald RD. CobraPLUS and Cookgas air-Q versus Fastrach for blind endotracheal intubation: a randomised controlled trial. Eur J Anaesthesiol 2011; 28: 181-6.
Hamard F, Ferrandiere M, Sauvagnac X, et al. Propofol sedation allows awake intubation of the difficult airway with the Fastrach™ LMA (French). Can J Anesth 2005; 52: 421-7.
Staikou C, Tsaroucha A, Paraskeva A, Fassoulaki A. Association between factors predicting and assessing the airway and use of intubating laryngeal mask airway. Middle East J Anesthesiol 2010; 20: 553-8.
Ydemann M, Rovsing L, Lindekaer AL, Olsen KS. Intubation of the morbidly obese patient: GlideScope® vs. Fastrach™. Acta Anaesthesiol Scand 2012; 56: 755-61.
Combes X, Sauvat S, Leroux B, et al. Intubating laryngeal mask airway in morbidly obese and lean patients: a comparative study. Anesthesiology 2005; 102: 1106-9.
Asai T, Eguchi Y, Murao K, Niitsu T, Shingu K. Intubating laryngeal mask for fibreoptic intubation—particularly useful during neck stabilization. Can J Anesth 2000; 47: 843-8.
Asai T, Shingu K. Tracheal intubation through the intubating laryngeal mask in patients with unstable necks. Acta Anaesthesiol Scand 2001; 45: 818-22.
Jagannathan N, Roth AG, Sohn LE, Pak TY, Amin S, Suresh S. The new air-Q intubating laryngeal airway for tracheal intubation in children with anticipated difficult airway: a case series. Paediatr Anaesth 2009; 19: 618-22.
Jagannathan N, Kho MF, Kozlowski RJ, Sohn LE, Siddique A, Wong DT. Retrospective audit of the air-Q intubating laryngeal airway as a conduit for tracheal intubation in pediatric patients with a difficult airway. Paediatr Anaesth 2011; 21: 422-7.
Theiler L, Kleine-Brueggeney M, Urwyler N, Graf T, Luyet C, Greif R. Randomized clinical trial of the i-gel™ and Magill tracheal tube or single-use ILMA™ and ILMA™ tracheal tube for blind intubation in anaesthetized patients with a predicted difficult airway. Br J Anaesth 2011; 107: 243-50.
Berkow LC, Schwartz JM, Kan K, Corridore M, Heitmiller ES. Use of the Laryngeal Mask Airway-Aintree Intubating Catheter-fiberoptic bronchoscope technique for difficult intubation. J Clin Anesth 2011; 23: 534-9.
van Zundert TC, Wong DT, van Zundert AA. The LMA-Supreme™ as an intubation conduit in patients with known difficult airways: a prospective evaluation study. Acta Anaesthesiol Scand 2013; 57: 77-81.
Cook TM, Seller C, Gupta K, Thornton M, O’Sullivan E. Non-conventional uses of the Aintree Intubating Catheter in management of the difficult airway. Anaesthesia 2007; 62: 169-74.
Higgs A, Clark E, Premraj K. Low-skill fibreoptic intubation: use of the Aintree Catheter with the classic LMA. Anaesthesia 2005; 60: 915-20.
Hung OR, Pytka S, Morris I, Murphy M, Stewart RD. Lightwand intubation: II—Clinical trial of a new lightwand for tracheal intubation in patients with difficult airways. Can J Anaesth 1995; 42: 826-30.
Rhee KY, Lee JR, Kim J, Park S, Kwon WK, Han S. A comparison of lighted stylet (Surch-Lite™) and direct laryngoscopic intubation in patients with high Mallampati scores. Anesth Analg 2009; 108: 1215-9.
Harvey K, Davies R, Evans A, Latto IP, Hall JE. A comparison of the use of Trachlight and Eschmann multiple-use introducer in simulated difficult intubation. Eur J Anaesthesiol 2007; 24: 76-81.
Turkstra TP, Craen RA, Pelz DM, Gelb AW. Cervical spine motion: a fluoroscopic comparison during intubation with lighted stylet, GlideScope, and Macintosh laryngoscope. Anesth Analg 2005; 101: 910-5.
Cooper RM, Pacey JA, Bishop MJ, McCluskey SA. Early clinical experience with a new videolaryngoscope (GlideScope®) in 728 patients. Can J Anesth 2005; 52: 191-8.
Hsiao WT, Lin YH, Wu HS, Chen CL. Does a new videolaryngoscope (glidescope) provide better glottic exposure? Acta Anaesthesiol Taiwan 2005; 43: 147-51.
Sun DA, Warriner CB, Parsons DG, Klein R, Umedaly HS, Moult M. The GlideScope® Video Laryngoscope: randomized clinical trial in 200 patients. Br J Anaesth 2005; 94: 381-4.
Siu LW, Mathieson E, Naik VN, Chandra D, Joo HS. Patient- and operator-related factors associated with successful Glidescope intubations: a prospective observational study in 742 patients. Anaesth Intensive Care 2010; 38: 70-5.
Griesdale DE, Liu D, McKinney J, Choi PT. Glidescope® video-laryngoscopy versus direct laryngoscopy for endotracheal intubation: a systematic review and meta-analysis. Can J Anesth 2012; 59: 41-52.
Serocki G, Bein B, Scholz J, Dorges V. Management of the predicted difficult airway: a comparison of conventional blade laryngoscopy with video-assisted blade laryngoscopy and the GlideScope. Eur J Anaesthesiol 2010; 27: 24-30.
Stroumpoulis K, Pagoulatou A, Violari M, et al. Videolaryngoscopy in the management of the difficult airway: a comparison with the Macintosh blade. Eur J Anaesthesiol 2009; 26: 218-22.
Malik MA, Subramaniam R, Maharaj CH, Harte BH, Laffey JG. Randomized controlled trial of the Pentax AWS, Glidescope, and Macintosh laryngoscopes in predicted difficult intubation. Br J Anaesth 2009; 103: 761-8.
Xue FS, Zhang GH, Liu J, et al. The clinical assessment of Glidescope in orotracheal intubation under general anesthesia. Minerva Anestesiol 2007; 73: 451-7.
Mosier JM, Stolz U, Chiu S, Sakles JC. Difficult airway management in the emergency department: GlideScope video laryngoscopy compared to direct laryngoscopy. J Emerg Med 2012; 42: 629-34.
Malik MA, Maharaj CH, Harte BH, Laffey JG. Comparison of Macintosh, Truview EVO2, Glidescope, and Airwayscope laryngoscope use in patients with cervical spine immobilization. Br J Anaesth 2008; 101: 723-30.
Phua DS, Mah CL, Wang CF. The Shikani optical stylet as an alternative to the GlideScope® videolaryngoscope in simulated difficult intubations—a randomised controlled trial. Anaesthesia 2012; 67: 402-6.
Agro F, Barzoi G, Montecchia F. Tracheal intubation using a Macintosh laryngoscope or a GlideScope in 15 patients with cervical spine immobilization. Br J Anaesth 2003; 90: 705-6.
Liu EH, Goy RW, Tan BH, Asai T. Tracheal intubation with videolaryngoscopes in patients with cervical spine immobilization: a randomized trial of the Airway Scope® and the GlideScope®. Br J Anaesth 2009; 103: 446-51.
Bathory I, Frascarolo P, Kern C, Schoettker P. Evaluation of the GlideScope® for tracheal intubation in patients with cervical spine immobilisation by a semi-rigid collar. Anaesthesia 2009; 64: 1337-41.
Lim Y, Yeo SW. A comparison of the GlideScope with the Macintosh laryngoscope for tracheal intubation in patients with simulated difficult airway. Anaesth Intensive Care 2005; 33: 243-7.
Lai HY, Chen IH, Chen A, Hwang FY, Lee Y. The use of the GlideScope® for tracheal intubation in patients with ankylosing spondylitis. Br J Anaesth 2006; 97: 419-22.
Huang SJ, Lee CL, Wang PK, Lin PC, Lai HY. The use of the GlideScope® for tracheal intubation in patients with halo vest. Acta Anaesthesiol Taiwan 2011; 49: 88-90.
Maassen R, Lee R, Hermans B, Marcus M, van Zundert A. A comparison of three videolaryngoscopes: the Macintosh laryngoscope blade reduces, but does not replace, routine stylet use for intubation in morbidly obese patients. Anesth Analg 2009; 109: 1560-5.
Andersen LH, Rovsing L, Olsen KS. GlideScope videolaryngoscope vs. Macintosh direct laryngoscope for intubation of morbidly obese patients: a randomized trial. Acta Anaesthesiol Scand 2011; 55: 1090-7.
Putz L, Dangelser G, Constant B, et al. Prospective trial comparing Airtraq™ and Glidescope™ techniques for intubation of obese patients (French). Ann Fr Anesth Reanim 2012; 31: 421-6.
Marrel J, Blanc C, Frascarolo P, Magnusson L. Videolaryngoscopy improves intubation condition in morbidly obese patients. Eur J Anaesthesiol 2007; 24: 1045-9.
Abdelmalak BB, Bernstein E, Egan C, et al. GlideScope® vs flexible fibreoptic scope for elective intubation in obese patients. Anaesthesia 2011; 66: 550-5.
Moore AR, Schricker T, Court O. Awake videolaryngoscopy-assisted tracheal intubation of the morbidly obese. Anaesthesia 2012; 67: 232-5.
Lange M, Frommer M, Redel A, et al. Comparison of the Glidescope® and Airtraq® optical laryngoscopes in patients undergoing direct microlaryngoscopy. Anaesthesia 2009; 64: 323-8.
Noppens RR, Mobus S, Heid F, Schmidtmann I, Werner C, Peipho T. Evaluation of the McGrath Series 5 videolaryngoscope after failed direct laryngoscopy. Anaesthesia 2010; 65: 716-20.
O’Leary AM, Sandison MR, Myneni N, et al. Preliminary evaluation of a novel videolaryngoscope, the McGrath series 5, in the management of difficult and challenging endotracheal intubation. J Clin Anesth 2008; 20: 320-1.
Ng I, Sim XL, Williams D, Segal R. A randomised controlled trial comparing the McGrath® videolaryngoscope with the straight blade laryngoscope when used in adult patients with potential difficult airways. Anaesthesia 2011; 66: 709-14.
Ng I, Hill AL, Williams DL, Lee K, Segal R. Randomized controlled trial comparing the McGrath videolaryngoscope with the C-MAC videolaryngoscope in intubating adult patients with potential difficult airways. Br J Anaesth 2012; 109: 439-43.
Rosenstock CV, Thogersen B, Afshari A, Christensen AL, Eriksen C, Gatke MR. Awake fiberoptic or awake video laryngoscopic tracheal intubation in patients with anticipated difficult airway management: a randomized clinical trial. Anesthesiology 2012; 116: 1210-6.
Taylor AM, Peck M, Launcelott S, et al. The McGrath® Series 5 videolaryngoscope vs the Macintosh laryngoscope: a randomised, controlled trial in patients with a simulated difficult airway. Anaesthesia 2013; 68: 142-7.
Piepho T, Fortmueller K, Heid FM, Schmidtmann I, Werner C, Noppens RR. Performance of the C-MAC video laryngoscope in patients after a limited glottic view using Macintosh laryngoscopy. Anaesthesia 2011; 66: 1101-5.
Aziz MF, Dillman D, Fu R, Brambrink AM. Comparative effectiveness of the C-MAC video laryngoscope versus direct laryngoscopy in the setting of the predicted difficult airway. Anesthesiology 2012; 116: 629-36.
Cavus E, Neumann T, Doerges V, et al. First clinical evaluation of the C-MAC D-Blade videolaryngoscope during routine and difficult intubation. Anesth Analg 2011; 112: 382-5.
Serocki G, Neumann T, Scharf E, Dorges V, Cavus E. Indirect videolaryngoscopy with C-MAC D-Blade and GlideScope: a randomized, controlled comparison in patients with suspected difficult airways. Minerva Anestesiol 2013; 79: 121-9.
Suzuki A, Toyama Y, Katsumi N, et al. The Pentax-AWS® rigid indirect video laryngoscope: clinical assessment of performance in 320 cases. Anaesthesia 2008; 63: 641-7.
Asai T, Liu EH, Matsumoto S, et al. Use of the Pentax-AWS in 293 patients with difficult airways. Anesthesiology 2009; 110: 898-904.
Hirabayashi Y, Seo N. Airway Scope: early clinical experience in 405 patients. J Anesth 2008; 22: 81-5.
Asai T, Enomoto Y, Okuda Y. Airway Scope for difficult intubation. Anaesthesia 2007; 62: 199.
Enomoto Y, Asai T, Arai T, Kamishima K, Okuda Y. Pentax-AWS, a new videolaryngoscope, is more effective than the Macintosh laryngoscope for tracheal intubation in patients with restricted neck movements: a randomized comparative study. Br J Anaesth 2008; 100: 544-8.
Komatsu R, Kamata K, Hoshi I, Sessler DI, Ozaki M. Airway scope and gum elastic bougie with Macintosh laryngoscope for tracheal intubation in patients with simulated restricted neck mobility. Br J Anaesth 2008; 101: 863-9.
Malin E, de Montblanc J, Ynineb Y, Marret E, Bonnet F. Performance of the Airtraq™ laryngoscope after failed conventional tracheal intubation: a case series. Acta Anaesthesiol Scand 2009; 53: 858-63.
Maharaj CH, Costello JF, Harte BH, Laffey JG. Evaluation of the Airtraq® and Macintosh laryngoscopes in patients at increased risk for difficult tracheal intubation. Anaesthesia 2008; 63: 182-8.
Maharaj CH, Costello JF, McDonnell JG, Harte BH, Laffey JG. The Airtraq® as a rescue airway device following failed direct laryngoscopy: a case series. Anaesthesia 2007; 62: 598-601.
Heidegger T, Gerig HJ, Ulrich B, Kreienbuhl G. Validation of a simple algorithm for tracheal intubation: daily practice is the key to success in emergencies—an analysis of 13,248 intubations. Anesth Analg 2001; 92: 517-22.
Heidegger T, Gerig HJ, Ulrich B, Schnider TW. Structure and process quality illustrated by fibreoptic intubation: analysis of 1612 cases. Anaesthesia 2003; 58: 734-9.
Xue FS, Liu HP, He N, et al. Spray-as-you-go airway topical anesthesia in patients with a difficult airway: a randomized, double-blind comparison of 2% and 4% lidocaine. Anesth Analg 2009; 108: 536-43.
Fuchs G, Schwarz G, Baumgartner A, Kaltenbock F, Voit-Augustin H, Planinz W. Fiberoptic intubation in 327 neurosurgical patients with lesions of the cervical spine. J Neurosurg Anesthesiol 1999; 11: 11-6.
Mihai R, Blair E, Kay H, Cook TM. A quantitative review and meta-analysis of performance of non-standard laryngoscopes and rigid fibreoptic intubation aids. Anaesthesia 2008; 63: 745-60.
Kim JK, Kim JA, Kim CS, Ahn HJ, Yang MK, Choi SJ. Comparison of tracheal intubation with the Airway Scope or Clarus Video System in patients with cervical collars. Anaesthesia 2011; 66: 694-8.
Bein B, Yan M, Tonner PH, Scholz J, Steinfath M, Dorges V. Tracheal intubation using the Bonfils intubation fibrescope after failed direct laryngoscopy. Anaesthesia 2004; 59: 1207-9.
Rudolph C, Henn-Beilharz A, Gottschall R, Wallenborn J, Schaffranietz L. The unanticipated difficult intubation: rigid or flexible endoscope? Minerva Anestesiol 2007; 73: 567-74.
Kim SH, Woo SJ, Kim JH. A comparison of Bonfils intubation fiberscopy and fiberoptic bronchoscopy in difficult airways assisted with direct laryngoscopy. Korean J Anesthesiol 2010; 58: 249-55.
Corbanese U, Possamai C. Awake intubation with the Bonfils fibrescope in patients with difficult airway. Eur J Anaesthesiol 2009; 26: 837-41.
Mazeres JE, Lefranc A, Cropet C, et al. Evaluation of the Bonfils intubating fibrescope for predicted difficult intubation in awake patients with ear, nose and throat cancer. Eur J Anaesthesiol 2011; 28: 646-50.
He N, Xue FS, Xu YC, Liao X, Xu XZ. Awake orotracheal intubation under airway topical anesthesia using the Bonfils in patients with a predicted difficult airway. Can J Anesth 2008; 55: 881-2.
Turkstra TP, Pelz DM, Shaikh AA, Craen RA. Cervical spine motion: a fluoroscopic comparison of Shikani Optical Stylet® vs Macintosh laryngoscope. Can J Anesth 2007; 54: 441-7.
Jabre P, Avenel A, Combes X, et al. Morbidity related to emergency endotracheal intubation—a substudy of the KETAmine SEDation trial. Resuscitation 2011; 82: 517-22.
Adnet F, Borron SW, Racine SX, et al. The intubation difficulty scale (IDS): proposal and evaluation of a new score characterizing the complexity of endotracheal intubation. Anesthesiology 1997; 87: 1290-7.
Jaber S, Amraoui J, Lefrant JY, et al. Clinical practice and risk factors for immediate complications of endotracheal intubation in the intensive care unit: a prospective, multiple-center study. Crit Care Med 2006; 34: 2355-61.
Mort TC. The incidence and risk factors for cardiac arrest during emergency tracheal intubation: a justification for incorporating the ASA Guidelines in the remote location. J Clin Anesth 2004; 16: 508-16.
Le Tacon S, Wolter P, Rusterholtz T, et al. Complications of difficult tracheal intubations in a critical care unit (French). Ann Fr Anesth Reanim 2000; 19: 719-24.
Henderson JJ, Popat MT, Latto IP, Pearce AC; Difficult Airway Society. Difficult Airway Society guidelines for management of the unanticipated difficult intubation. Anaesthesia 2004; 59: 675-94.
Hamaekers AE, Henderson JJ. Equipment and strategies for emergency tracheal access in the adult patient. Anaesthesia 2011; 66(Suppl 2): 65-80.
Parmet JL, Colonna-Romano P, Horrow JC, Miller F, Gonzales J, Rosenberg H. The laryngeal mask airway reliably provides rescue ventilation in cases of unanticipated difficult tracheal intubation along with difficult mask ventilation. Anesth Analg 1998; 87: 661-5.
Mort TC. Laryngeal mask airway and bougie intubation failures: the Combitube as a secondary rescue device for in-hospital emergency airway management. Anesth Analg 2006; 103: 1264-6.
Keller C, Brimacombe J, Lirk P, Puhringer F. Failed obstetric tracheal intubation and postoperative respiratory support with the ProSeal™ laryngeal mask airway. Anesth Analg 2004; 98: 1467-70.
Della Puppa A, Pittoni G, Frass M. Tracheal esophageal combitube: a useful airway for morbidly obese patients who cannot intubate or ventilate. Acta Anaesthesiol Scand 2002; 46: 911-3.
Lim CL, Hawthorne L, Ip-Yam PC. The intubating laryngeal mask airway (ILMA) in failed and difficult intubation. Anaesthesia 1998; 53: 929-30.
Lomax SL, Loveland R, Rangasami J. Can’t intubate, can’t ventilate: rescue airway using a size 2(1/2) laryngeal mask airway in a morbidly obese female patient. Eur J Anaesthesiol 2009; 26: 1090-1.
Matioc AA, Olson J. Use of the Laryngeal Tube™ in two unexpected difficult airway situations: lingual tonsillar hyperplasia and morbid obesity. Can J Anesth 2004; 51: 1018-21.
Boisson-Bertrand D, Bourgain JL, Camboulives J, et al. Intubation difficile. Ann Fr Anesth Reanim 1996; 15: 207-14.
Petrini F, Accorsi A, Adrario E, et al. Recommendations for airway control and difficult airway management. Minerva Anestesiol 2005; 71: 617-57.
Braude D, Webb H, Stafford J, et al. The bougie-aided cricothyrotomy. Air Med J 2009; 28: 191-4.
Hubble MW, Wilfong DA, Brown LH, Hertelendy A, Benner RW. A meta-analysis of prehospital airway control techniques part II: alternative airway devices and cricothyrotomy success rates. Prehosp Emerg Care 2010; 14: 515-30.
Wong DT, Lai K, Chung FF, Ho RY. Cannot intubate-cannot ventilate and difficult intubation strategies: results of a Canadian national survey. Anesth Analg 2005; 100: 1439-46.
Elliott DS, Baker PA, Scott MR, Birch CW, Thompson JM. Accuracy of surface landmark identification for cannula cricothyroidotomy. Anaesthesia 2010; 65: 889-94.
Aslani A, Ng SC, Hurley M, McCarthy KF, McNicholas M, McCaul CL. Accuracy of identification of the cricothyroid membrane in female subjects using palpation: an observational study. Anesth Analg 2012; 114: 987-92.
Cook TM, Nolan JP. Use of capnography to confirm correct tracheal intubation during cardiac arrest. Anaesthesia 2011; 66: 1183-4.
Merchant R, Chartrand D, Dain S, et al. Guidelines to the practice of anesthesia revised edition 2013. Can J Anesth 2013; 60: 60-84.
Quinn AC, Milne D, Columb M, Gorton H, Knight M. Failed tracheal intubation in obstetric anaesthesia: 2 yr national case-control study in the UK. Br J Anaesth 2013; 110: 74-80.
Djabatey EA, Barclay PM. Difficult and failed intubation in 3430 obstetric general anaesthetics. Anaesthesia 2009; 64: 1168-71.
McKeen DM, George RB, O’Connell CM, et al. Difficult and failed intubation: incident rates and maternal, obstetrical, and anesthetic predictors. Can J Anesth 2011; 58: 514-24.
Douglas MJ, Preston RL. The obstetric airway: things are seldom as they seem. Can J Anesth 2011; 58: 494-8.
Russell IF, Chambers WA. Closing volume in normal pregnancy. Br J Anaesth 1981; 53: 1043-7.
McClelland SH, Bogod DG, Hardman JG. Pre-oxygenation and apnoea in pregnancy: changes during labour and with obstetric morbidity in a computational simulation. Anaesthesia 2009; 64: 371-7.
Hignett R, Fernando R, McGlennan A, et al. A randomized crossover study to determine the effect of a 30° head-up versus a supine position on the functional residual capacity of term parturients. Anesth Analg 2011; 113: 1098-102.
Izci B, Riha RL, Martin SE, et al. The upper airway in pregnancy and pre-eclampsia. Am J Respir Crit Care Med 2003; 167: 137-40.
Kodali BS, Chandrasekhar S, Bulich LN, Topulos GP, Datta S. Airway changes during labor and delivery. Anesthesiology 2008; 108: 357-62.
Boutonnet M, Faitot V, Katz A, Salomon L, Keita H. Mallampati class changes during pregnancy, labour, and after delivery: can these be predicted? Br J Anaesth 2010; 104: 67-70.
Norris AM, Hardman JG, Asai T. A firm foundation for progress in airway management. Br J Anaesth 2011; 106: 613-6.
Tsen LC, Pitner R, Camann WR. General anesthesia for cesarean section at a tertiary care hospital 1990-1995: indications and implications. Int J Obstet Anesth 1998; 7: 147-52.
Palanisamy A, Mitani AA, Tsen LC. General anesthesia for cesarean delivery at a tertiary care hospital from 2000 to 2005: a retrospective analysis and 10-year update. Int J Obstet Anesth 2011; 20: 10-6.
Wong SH, Hung CT. Prevalence and prediction of difficult intubation in Chinese women. Anaesth Intensive Care 1999; 27: 49-52.
Honarmand A, Safavi MR. Prediction of difficult laryngoscopy in obstetric patients scheduled for caesarean delivery. Eur J Anaesthesiol 2008; 25: 714-20.
Basaranoglu G, Columb M, Lyons G. Failure to predict difficult tracheal intubation for emergency caesarean section. Eur J Anaesthesiol 2010; 27: 947-9.
Collins JS, Lemmens HJ, Brodsky JB, Brock-Utne JG, Levitan RM. Laryngoscopy and morbid obesity: a comparison of the “sniff” and “ramped” positions. Obes Surg 2004; 14: 1171-5.
Chiron B, Laffon M, Ferrandiere M, Pittet JF, Marret H, Mercier C. Standard preoxygenation technique versus two rapid techniques in pregnant patients. Int J Obstet Anesth 2004; 13: 11-4.
El-Orbany M, Connolly LA. Rapid sequence induction and intubation: current controversy. Anesth Analg 2010; 110: 1318-25.
Habib AS. Is it time to revisit tracheal intubation for cesarean delivery? Can J Anesth 2012; 59: 642-7.
Hawkins JL, Chang J, Palmer SK, Gibbs CP, Callaghan WM. Anesthesia-related maternal mortality in the United States: 1979-2002. Obstet Gynecol 2011; 117: 69-74.
McClure JH, Cooper GM, Clutton-Brock TH, Centre for Maternal and Child Enquiries. Saving mothers’ lives: reviewing maternal deaths to make motherhood safer: 2006-8: a review. Br J Anaesth 2011; 107: 127-32.
Bhananker SM, Ramamoorthy C, Geiduschek JM, et al. Anesthesia-related cardiac arrest in children: update from the Pediatric Perioperative Cardiac Arrest Registry. Anesth Analg 2007; 105: 344-50.
Murat I, Constant I, Maud’huy H. Perioperative anaesthetic morbidity in children: a database of 24,165 anaesthetics over a 30-month period. Paediatr Anaesth 2004; 14: 158-66.
Heinrich S, Birkholz T, Ihmsen H, Irouschek A, Ackermann A, Schmidt J. Incidence and predictors of difficult laryngoscopy in 11,219 pediatric anesthesia procedures. Paediatr Anaesth 2012; 22: 729-36.
Weiss M, Engelhardt T. Proposal for the management of the unexpected difficult pediatric airway. Paediatr Anaesth 2010; 20: 454-64.
Vlatten A, Aucoin S, Litz S, Macmanus B, Soder C. A comparison of the STORZ video laryngoscope and standard direct laryngoscopy for intubation in the pediatric airway—a randomized clinical trial. Paediatr Anaesth 2009; 19: 1102-7.
Macnair D, Baraclough D, Wilson G, Bloch M, Engelhardt T. Pediatric airway management: comparing the Berci-Kaplan Video Laryngoscope with direct laryngoscopy. Paediatr Anaesth 2009; 19: 577-80.
Kim JT, Na HS, Bae JY, et al. GlideScope® video laryngoscope: a randomized clinical trial in 203 paediatric patients. Br J Anaesth 2008; 101: 531-4.
Fiadjoe JE, Gurnaney H, Dalesio N, et al. A prospective randomized equivalence trial of the GlideScope Cobalt® video laryngoscope to traditional direct laryngoscopy in neonates and infants. Anesthesiology 2012; 116: 622-8.
Armstrong J, John J, Karsli C. A comparison between the GlideScope Video Laryngoscope and direct laryngoscope in paediatric patients with difficult airways – a pilot study. Anaesthesia 2010; 65: 353-7.
Weiss M, Dullenkopf A, Fischer JE, Keller C, Gerber AC, European Paediatric Endotracheal Intubation Study Group. Prospective randomized controlled multi-centre trial of cuffed or uncuffed endotracheal tubes in small children. Br J Anaesth 2009; 103: 867-73.
Newth CJ, Rachman B, Patel N, Hammer J. The use of cuffed versus uncuffed endotracheal tubes in pediatric intensive care. J Pediatr 2004; 144: 333-7.
Dorsey DP, Bowman SM, Klein MB, Archer D, Sharar SR. Perioperative use of cuffed endotracheal tubes is advantageous in young pediatric burn patients. Burns 2010; 36: 856-60.
Gopalareddy V, He Z, Soundar S, et al. Assessment of the prevalence of microaspiration by gastric pepsin in the airway of ventilated children. Acta Paediatr 2008; 97: 55-60.
Walker RW. The laryngeal mask airway in the difficult paediatric airway: an assessment of positioning and use in fibreoptic intubation. Paediatr Anaesth 2000; 10: 53-8.
Bahk JH, Han SM, Kim SD. Management of difficult airways with a laryngeal mask airway under propofol anaesthesia. Paediatr Anaesth 1999; 9: 163-6.
Selim M, Mowafi H, Al-Ghamdi A, Adu-Gyamfi Y. Intubation via LMA in pediatric patients with difficult airways. Can J Anesth 1999; 46: 891-3.
Cote CJ, Hartnick CJ. Pediatric transtracheal and cricothyrotomy airway devices for emergency use: which are appropriate for infants and children? Paediatr Anaesth 2009; 19(Suppl 1): 66-76.
Metterlein T, Frommer M, Kwok P, Lyer S, Graf BM, Sinner B. Emergency cricothyrotomy in infants—evaluation of a novel device in an animal model. Paediatr Anaesth 2011; 21: 104-9.
Smith RB, Schaer WB, Pfaeffle H. Percutaneous transtracheal ventilation for anaesthesia and resuscitation: a review and report of complications. Can Anaesth Soc J 1975; 22: 607-12.
Auden SM. Additional techniques for managing the difficult pediatric airway. Anesth Analg 2000; 90: 878-80.
Bair AE, Filbin MR, Kulkarni RG, Walls RM. The failed intubation attempt in the emergency department: analysis of prevalence, rescue techniques, and personnel. J Emerg Med 2002; 23: 131-40.
Santoro AS, Cooper MG, Cheng A. Failed intubation and failed oxygenation in a child. Anaesth Intensive Care 2012; 40: 1056-8.
Bourgain JL, Desruennes E, Fischler M, Ravussin P. Transtracheal high frequency jet ventilation for endoscopic airway surgery: a multicentre study. Br J Anaesth 2001; 87: 870-5.
Jaquet Y, Monnier P, Van Melle G, Ravussin P, Spahn DR, Chollet-Rivier M. Complications of different ventilation strategies in endoscopic laryngeal surgery: a 10-year review. Anesthesiology 2006; 104: 52-9.
Depierraz B, Ravussin P, Brossard E, Monnier P. Percutaneous transtracheal jet ventilation for paediatric endoscopic laser treatment of laryngeal and subglottic lesions. Can J Anaesth 1994; 41: 1200-7.
Preussler NP, Schreiber T, Huter L, et al. Percutaneous transtracheal ventilation: effects of a new oxygen flow modulator on oxygenation and ventilation in pigs compared with a hand triggered emergency jet injector. Resuscitation 2003; 56: 329-33.
Yildiz Y, Preussler NP, Schreiber T, et al. Percutaneous transtracheal emergency ventilation during respiratory arrest: comparison of the oxygen flow modulator with a hand-triggered emergency jet injector in an animal model. Am J Emerg Med 2006; 24: 455-9.
Baker PA, Brown AJ. Experimental adaptation of the Enk oxygen flow modulator for potential pediatric use. Paediatr Anaesth 2009; 19: 458-63.
Metz S, Parmet JL, Levitt JD. Failed emergency transtracheal ventilation through a 14-gauge intravenous catheter. J Clin Anesth 1996; 8: 58-62.
Arne J, Descoins P, Fusciardi J, et al. Preoperative assessment for difficult intubation in general and ENT surgery: predictive value of a clinical multivariate risk index. Br J Anaesth 1998; 80: 140-6.
Lundstrom LH, Moller AM, Rosenstock C, et al. A documented previous difficult tracheal intubation as a prognostic test for a subsequent difficult tracheal intubation in adults. Anaesthesia 2009; 64: 1081-8.
Cook T, Woodall N, Frerk C. 4th National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Major complications of airway management in the United Kingdom. London: The Royal College of Anaesthetists; 2011.
Gaba DM, Howard SK, Flanagan B, Smith BE, Fish KJ, Botney R. Assessment of clinical performance during simulated crises using both technical and behavioral ratings. Anesthesiology 1998; 89: 8-18.
Fletcher G, Flin R, McGeorge P, Glavin R, Maran N, Patey R. Anaesthetists’ Non-Technical Skills (ANTS): evaluation of a behavioural marker system. Br J Anaesth 2003; 90: 580-8.
Leblanc VR. Review article: simulation in anesthesia: state of the science and looking forward. Can J Anesth 2012; 59: 193-202.
Boet S, Bould MD, Schaeffer R, et al. Learning fibreoptic intubation with a virtual computer program transfers to ‘hands on’ improvement. Eur J Anaesthesiol 2010; 27: 31-5.
Friedman Z, You-Ten KE, Bould MD, Naik V. Teaching lifesaving procedures: the impact of model fidelity on acquisition and transfer of cricothyrotomy skills to performance on cadavers. Anesth Analg 2008; 107: 1663-9.
Naik VN, Matsumoto ED, Houston PL, et al. Fiberoptic orotracheal intubation on anesthetized patients: do manipulation skills learned on a simple model transfer into the operating room? Anesthesiology 2001; 95: 343-8.
Chandra DB, Savoldelli GL, Joo HS, Weiss ID, Naik VN. Fiberoptic oral intubation: the effect of model fidelity on training for transfer to patient care. Anesthesiology 2008; 109: 1007-13.
Komatsu R, Kasuya Y, Yogo H, et al. Learning curves for bag-and-mask ventilation and orotracheal intubation: an application of the cumulative sum method. Anesthesiology 2010; 112: 1525-31.
Plummer JL, Owen H. Learning endotracheal intubation in a clinical skills learning center: a quantitative study. Anesth Analg 2001; 93: 656-62.
Savoldelli GL, Schiffer E, Abegg C, Baeriswyl V, Clergue F, Waeber JL. Learning curves of the Glidescope, the McGrath and the Airtraq laryngoscopes: a manikin study. Eur J Anaesthesiol 2009; 26: 554-8.
Wong DT, Prabhu AJ, Coloma M, Imasogie N, Chung FF. What is the minimum training required for successful cricothyroidotomy?: a study in mannequins. Anesthesiology 2003; 98: 349-53.
Yee B, Naik VN, Joo HS, et al. Nontechnical skills in anesthesia crisis management with repeated exposure to simulation-based education. Anesthesiology 2005; 103: 241-8.
Sullivan ME, Brown CV, Peyre SE, et al. The use of cognitive task analysis to improve the learning of percutaneous tracheostomy placement. Am J Surg 2007; 193: 96-9.
Nishisaki A, Nguyen J, Colborn S, et al. Evaluation of multidisciplinary simulation training on clinical performance and team behavior during tracheal intubation procedures in a pediatric intensive care unit. Pediatr Crit Care Med 2011; 12: 406-14.
Siu LW, Boet S, Borges BC, et al. High-fidelity simulation demonstrates the influence of anesthesiologists’ age and years from residency on emergency cricothyroidotomy skills. Anesth Analg 2010; 111: 955-60.
Graham CA. Advanced airway management in the emergency department: what are the training and skills maintenance needs for UK emergency physicians? Emerg Med J 2004; 21: 14-9.
Boet S, Borges BC, Naik VN, et al. Complex procedural skills are retained for a minimum of 1 yr after a single high-fidelity simulation training session. Br J Anaesth 2011; 107: 533-9.
Acknowledgements
The authors sincerely thank Drs. Narasimhan Jagannathan, Stephan Malherbe, Vito Forte, and Lawrence Roy for their additional contributions in support of this project. Supported in part by the Department of Anesthesia, Dalhousie University.
Conflicts of interest
Dr. J. Adam Law is co-director of and royalty recipient from the Airway Interventions and Management in Emergencies (AIME) course and a recipient of equipment (as loan or gift) from Ambu A/S.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
(Please see Appendix 2 for authors’ affiliations, attributions, and disclosures).
This article is accompanied by an editorial. Please see Can J Anesth 2013; 60: this issue.
Appendices
Appendix 1
Sample template of a difficult airway alert letter: to be given to the patient following a difficult airway encounter, with copies to the hospital chart and the primary care provider. Modified from a previously published example.12


Appendix 2: Authorship affiliations, attribution, and disclosures
Authors | Affiliations |
|---|---|
J. Adam Law, MD | Department of Anesthesia, Pain Management and Perioperative Medicine, Queen Elizabeth II Health Sciences Centre; Dalhousie University. 1796 Summer Street, Halifax, Nova Scotia B3H 3A7, Canada. E-mail: jlaw@dal.ca |
Natasha Broemling, MD | Department of Pediatric Anesthesia, BC Children’s Hospital; University of British Columbia |
Richard M. Cooper, MD | Department of Anesthesia and Pain Management, University Health Network, Toronto General Hospital Site; University of Toronto |
Pierre Drolet, MD | Département d’anesthésiologie, Hôpital Maisonneuve-Rosemont; Université de Montréal |
Laura V. Duggan, MD | Department of Anesthesiology, Pharmacology and Therapeutics, Royal Columbian Hospital; University of British Columbia |
Donald E. Griesdale, MD, MPH | a. Department of Anesthesia, Pharmacology and Therapeutics, University of British Columbia, Vancouver BC, Canada b. Department of Medicine, Division of Critical Care Medicine, University of British Columbia, Vancouver BC, Canada c. Centre for Clinical Epidemiology and Evaluation, Vancouver Coastal Health Research Institute, Vancouver BC, Canada |
Orlando R. Hung, MD | Department of Anesthesia, Pain Management and Perioperative Medicine, Queen Elizabeth II Health Sciences Centre; Dalhousie University. |
Philip M. Jones, MD, MSc | Department of Anesthesia and Perioperative Medicine, University Hospital, London Health Sciences Centre; Western University |
George Kovacs, MD, MHPE | Department of Emergency Medicine, Queen Elizabeth II Health Sciences Centre; Dalhousie University. |
Simon Massey, MB, BCh | Department of Anesthesiology, Pharmacology and Therapeutics, BC Women’s Hospital and Health Centre; University of British Columbia |
Roanne Preston, MD | Department of Anesthesiology, Pharmacology and Therapeutics; Faculty of Medicine; University of British Columbia |
Ian R. Morris, MD | Department of Anesthesia, Pain Management and Perioperative Medicine, Queen Elizabeth II Health Sciences Centre; Dalhousie University. |
Timothy Mullen, MD | Department of Anesthesia, Pain Management and Perioperative Medicine, Queen Elizabeth II Health Sciences Centre; Dalhousie University. |
Michael F. Murphy, MD | Department of Anesthesiology and Pain Medicine, Walter Mackenzie Health Sciences Centre; University of Alberta |
Viren N. Naik, MD, MEd | Department of Anesthesiology, The Ottawa Hospital; University of Ottawa |
Jeanette Scott, MB, ChB, FANZCA | Middlemore Hospital, Auckland, New Zealand |
Shean Stacey, MD | Department of Anesthesia, Foothills Medical Centre; University of Calgary |
Timothy P. Turkstra, MD, MEng | Department of Anesthesia and Perioperative Medicine; Western University |
David T. Wong, MD | Department of Anesthesia, University Health Network, Toronto Western Hospital site; University of Toronto |
Authors | Attribution(s) | Disclosure(s) |
|---|---|---|
J. Adam Law MD | Focus group chair; data acquisition, analysis, and interpretation; writing and critically revising article. | Work supported by the Department of Anesthesia, Dalhousie University. Co-director of and royalty recipient from Airway Interventions and Management in Emergencies (AIME) course. Recipient of equipment (as loan or gift) from Ambu A/S. |
Natasha Broemling MD | Data acquisition, analysis, and interpretation; critically revising article. | None |
Richard M. Cooper MD | Data acquisition, analysis, and interpretation; writing and critically revising article. | Unpaid consultant to Verathon. Recipient of equipment (as loan or gift) from Clarus, McGrath, Prodol, Verathon, Karl Storz. |
Pierre Drolet, MD | Data acquisition, analysis, and interpretation; critically revising article. | None |
Laura V. Duggan MD | Data acquisition, analysis, and interpretation; writing and critically revising article. | None |
Donald E. Griesdale MD | Data acquisition, analysis, and interpretation; critically revising article. | Funding sources: Institutional: Clinician Scientist Award from the Vancouver Coastal Health Research Institute Departmental: Vancouver Hospital Department of Anesthesia |
Orlando R. Hung MD | Data acquisition, analysis, and interpretation; critically revising article. | Consultant to Covidien and King Systems |
Philip M. Jones MD | Data acquisition, analysis, and interpretation; critically revising article. | None |
George Kovacs MD | Data acquisition, analysis, and interpretation; critically revising article. | Co-director of and royalty recipient from Airway Interventions and Management in Emergencies (AIME) course. |
Simon Massey MB BCh | Data acquisition, analysis, and interpretation; critically revising article. | None |
Roanne Preston MD | Data acquisition, analysis, and interpretation; critically revising article. | None |
Ian R. Morris MD | Data acquisition, analysis, and interpretation; writing and critically revising article. | None |
Timothy Mullen MD | Data acquisition, analysis, and interpretation; critically revising article. | None |
Michael F. Murphy MD | Data acquisition, analysis, and interpretation; critically revising article. | Owner of Airway Management Education Center (the Difficult Airway Course™ Anesthesia and Emergency); and First Airway (The Difficult Airway Course: EMS™ and Fundamentals of Airway Management™) |
Viren N. Naik MD | Data acquisition, analysis, and interpretation; writing and critically revising article. | Work supported by University of Ottawa Skills and Simulation Centre |
Jeanette Scott MD | Data acquisition, analysis and interpretation; writing and critically revising article. | None |
Shean Stacey MD | Data acquisition, analysis, and interpretation; critically revising article. | None |
Timothy P. Turkstra MD | Data acquisition, analysis, and interpretation; writing and critically revising article. | None |
David T. Wong MD | Data acquisition, analysis, and interpretation; critically revising article. | Supported in part by the Department of Anesthesia, Toronto Western Hospital, University of Toronto |
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Law, J.A., Broemling, N., Cooper, R.M. et al. The difficult airway with recommendations for management – Part 1 – Difficult tracheal intubation encountered in an unconscious/induced patient. Can J Anesth/J Can Anesth 60, 1089–1118 (2013). https://doi.org/10.1007/s12630-013-0019-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-013-0019-3