Abstract
Progress in biomarker development has greatly enhanced our ability to categorize breast cancer into several clinical subtypes and to deliver better personalized therapies. Technological advances in gene expression profiling, signaling pathways, proliferation markers and tumor monitoring through detection of circulating tumor cells and free DNA, and measurements of genomic instability and germline mutations are being vigorously pursued in breast cancer research. Their application in routine clinical practice is increasing and helping further development of precision medicine. Ongoing challenges include assessing the utility and feasibility of these tests, interpreting the large amounts of genomic data that are being generated, translating the information to clinical practice, and constructing clinical trials on molecularly driven approaches. In this article, we will review current and emerging promising biomarkers and their roles in the management of patients with breast cancer.
Similar content being viewed by others
References
Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25(33):5287–312.
Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–52.
Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98(19):10869–74.
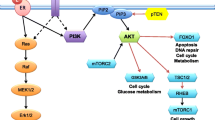
Kourea HP, Zolota V, Scopa CD. Targeted pathways in breast cancer: molecular and protein markers guiding therapeutic decisions. Curr Mol Pharmacol. 2014;7(1):4–21.
Massihnia D, Perez A, Bazan V, et al. A headlight on liquid biopsies: a challenging tool for breast cancer management. Tumour Biol. 2016 Jan 20.
Malvezzi M, Bertuccio P, Rosso T, Rota M, Levi F, La Vecchia C, et al. European cancer mortality predictions for the year 2015: does lung cancer have the highest death rate in EU women? Ann Oncol. 2015;26(4):779–86.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29.
Sharma K, Costas A, Shulman LN, Meara JG. A systematic review of barriers to breast cancer care in developing countries resulting in delayed patient presentation. J Oncol. 2012;2012:121873.
El Saghir NS, Khalil MK, Eid T, et al. Trends in epidemiology and management of breast cancer in developing Arab countries: a literature and registry analysis. Int J Surg. 2007;5(4):225–33.
Anderson BO, Ilbawi AM, El Saghir NS. Breast cancer in low and middle income countries (LMICs): a shifting tide in global health. Breast J. 2015;21(1):111–8.
World Health Organization. Cancer rates could increase by 50% to 15 million by 2020. WHO; 2010.
Rodríguez-Antona C, Taron M. Pharmacogenomic biomarkers for personalized cancer treatment. J Intern Med. 2015;277(2):201–17.
American Cancer Society. Cancer facts & figures 2012. American Cancer Society; 2012. p. 11.
Andre F, McShane LM, Michiels S, Ransohoff DF, Altman DG, Reis-Filho JS, et al. Biomarker studies: a call for a comprehensive biomarker study registry. Nat Rev Clin Oncol. 2011;8(3):171–6.
Diamandis EP. Cancer biomarkers: can we turn recent failures into success? J Natl Cancer Inst. 2010;102(19):1462–7.
Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;31(1):13–20.
Sotiriou C, Pusztai L. Gene-expression signatures in breast cancer. N Engl J Med. 2009;360(8):790–800.
Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thürlimann B, Senn HJ, et al. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol. 2009;20(8):1319–29.
Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010;11(2):174–83.
Jackisch C, Harbeck N, Huober J, et al. 14th St. Gallen International Breast Cancer Conference 2015: Evidence, controversies, consensus - primary therapy of early breast cancer: opinions expressed by German experts. Breast Care (Basel). 2015;10(3):211–9.
Coates AS, Winer EP, Goldhirsch A, et al. Panel Members. Tailoring therapies-improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol. 2015;26(8):1533–46.
de Azambuja E, Cardoso F, de Castro Jr G, et al. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer. 2007;96(10):1504–13.
Petrelli F, Viale G, Cabiddu M, et al. Prognostic value of different cut-off levels of Ki-67 in breast cancer: a systematic review and meta-analysis of 64,196 patients. Breast Cancer Res Treat. 2015;153(3):477–91.
Dowsett M, Smith IE, Ebbs SR, et al. Short-term changes in Ki-67 during neoadjuvant treatment of primary breast cancer with anastrozole or tamoxifen alone or combined correlate with recurrence-free survival. Clin Cancer Res. 2005;11:951–8.
Alba E, Lluch A, Ribelles N, et al. High proliferation predicts pathological complete response to neoadjuvant chemotherapy in early breast cancer. Oncologist. 2016 Jan 19. pii: theoncologist.2015-0312.
Criscitiello C, Disalvatore D, De Laurentiis M, et al. High Ki-67 score is indicative of a greater benefit from adjuvant chemotherapy when added to endocrine therapy in luminal B HER2 negative and node-positive breast cancer. Breast. 2014;23(1):69–75. doi:10.1016/j.breast.2013.11.007.
Ricciardi GRR, Adamo B, Ieni A, Licata L, Cardia R, Ferraor G, et al. Androgen Receptor (AR), E-Cadherin, and Ki-67 as emerging targets and novel prognostic markers in triple-negative breast cancer (TNBC) patients. PLoS ONE. 2015;10(6), e0128368.
Kim T, Han W, Kim MK, Lee JW, Kim J, Ahn SK, et al. Predictive significance of p53, Ki-67, and Bcl-2 expression for pathologic complete response after neoadjuvant chemotherapy for triple-negative breast cancer. J Breast Cancer. 2015;18(1):16–21.
Cheang MC, Chia SK, Voduc D, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101(10):736–50.
Dowsett M, Nielsen TO, A’Hern R, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011;103(22):1656–64.
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–82.
Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–12.
Hudis CA. Trastuzumab—mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51.
Press MF, Bernstein L, Thomas PA, et al. HER-2/neu gene amplification characterized by fluorescence in situ hybridization: poor prognosis in node-negative breast carcinomas. J Clin Oncol. 1997;15:2894–904.
Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92.
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–72.
Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–84.
Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–83.
Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABPB-31 and NCCTG N9831. J Clin Oncol. 2014;32(33):3744–52.
Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–85.
Untch M, Rezai M, Loibl S, et al. Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro study. J Clin Oncol. 2010;28:2024–31.
Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375(9712):377–84.
Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–43.
Di Leo A, Gomez HL, Aziz Z, et al. Phase III, double-blind, randomized study comparing lapatinib plus paclitaxel with placebo plus paclitaxel as first-line treatment for metastatic breast cancer. J Clin Oncol. 2008;26:5544–52.
Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379(9816):633–40.
Arribas J, Baselga J, Pedersen K, et al. p95HER2 and breast cancer. Cancer Res. 2011;71:1515–9.
Scaltriti M, Rojo F, Ocana A, et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst. 2007;99:628–38.
Pedersen K, Angelini PD, Laos S, et al. A naturally occurring HER2 carboxy-terminal fragment promotes mammary tumor growth and metastasis. Mol Cell Biol. 2009;29:3319–31.
Duchnowska R, Sperinde J, Chenna A, et al. Quantitative measurements of tumoral p95HER2 protein expression in metastatic breast cancer patients treated with trastuzumab: independent validation of the p95HER2 clinical cutoff. Clin Cancer Res. 2014;20:2805–13.
Loibl S, Bruey J, Von Minckwitz G, et al. Validation of p95 as a predictive marker for trastuzumab-based therapy in primary HER2-positive breast cancer: a translational investigation from the neoadjuvant GeparQuattro study. J Clin Oncol. 2011;29(Suppl):abstr 530.
Majewski IJ, Nuciforo P, Mittempergher L, et al. PIK3CA mutations are associated with decreased benefit to neoadjuvant human epidermal growth factor receptor 2-targeted therapies in breast cancer. J Clin Oncol. 2015;33(12):1334–9.
Baselga J, Cortes J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–19.
Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–91.
Carey LA, Berry DA, Cirrincione CT, et al. Molecular heterogeneity and response to neoadjuvant human epidermal growth factor receptor 2 targeting in CALGB 40601, a randomized phase III trial of paclitaxel plus trastuzumab with or without lapatinib. J Clin Oncol. 2015 Nov 2.
Loi S, Savas P. Looking deep into the heterogeneity of Human Epidermal Growth Factor Receptor 2 – Positive breast cancer: Can we understand it better? J Clin Oncol. Published online before print January 11, 2016.
Yang Z, Barnes CJ, Kumar R. Human epidermal growth factor receptor 2 status modulates subcellular localization of and interaction with estrogen receptor alpha in breast cancer cells. Clin Cancer Res. 2004;10(11):3621–8.
Parra-Palau JL, Pedersen K, Peg V, et al. A major role of p95/611-CTF, a carboxy-terminal fragment of HER2, in the down-modulation of the estrogen receptor in HER2-positive breast cancers. Cancer Res. 2010;70(21):8537–46.
Ithimakin S, Day KC, Malik F, et al. HER2 drives luminal breast cancer stem cells in the absence of HER2 amplification: implications for efficacy of adjuvant trastuzumab. Cancer Res. 2013;73:1635–46.
Bose R, Kavuri SM, Searleman AC, et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 2013;3:224–37.
Ciccolini J, Fanciullino R, Serdjebi C, Milano G. Pharmacogenetics and breast cancer management: current status and perspectives. Expert Opin Drug Metab Toxicol. 2015;11(5):719–29.
Bidard FC, Fehm T, Ignatiadis M, Smerage JB, Alix-Panabières C, Janni W, et al. Clinical application of circulating tumor cells in breast cancer: overview of the current interventional trials. Cancer Metastasis Rev. 2013;32(1–2):179–88.
Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88(3):323–31.
Allred DC, Clark GM, Elledge R, Fuqua SA, Brown RW, Chamness GC, et al. Association of p53 protein expression with tumor cell proliferation rate and clinical outcome in node-negative breast cancer. J Natl Cancer Inst. 1993;85(3):200–6.
Olivier M, Langerød A, Carrieri P, Bergh J, Klaar S, Eyfjord J, et al. The clinical value of somatic TP53 gene mutations in 1,794 patients with breast cancer. Clin Cancer Res. 2006;12(4):1157–67.
Dookeran KA, Dignam JJ, Ferrer K, Sekosan M, McCaskill-Stevens W, Gehlert S. p53 as a marker of prognosis in African American women with breast cancer. Ann Surg Oncol. 2010;17:1398–405.
Bozovic-Spasojevic IAmeye L, Paesmans M, Larsimont D, Di Leo A, Dolci S, et al. Prognostic, predictive abilities and concordance of BCL2 and TP53 protein expression in primary breast cancers and axillary lymph-nodes: a retrospective analysis of the Belgian three arm study evaluating anthracycline vs CMF adjuvant chemotherapy. Breast. 2014;23:473–81.
Soerjomataram I, Louwman MW, Ribot JG, Roukema JA, Coebergh JW. An overview of prognostic factors for long-term survivors of breast cancer. Breast Cancer Res Treat. 2008;107(3):309–30.
Bernards R. Reaction to American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2008;26(12):2057–8.
Hosokawa Y, Gadd M, Smith AP, Koerner FC, Schmidt EV, Arnold A. Cyclin D1 (PRAD1) alternative transcript b: full-length cDNA cloning and expression in breast cancers. Cancer Lett. 1997;113:123–30.
Arnold A, Papanikolaou A. Cyclin D1 in breast cancer pathogenesis. J Clin Oncol. 2005;23:4215–24.
Mohamed A, Krajewski K, Cakar B, Ma CX. Targeted therapy for breast cancer. Am J Pathol. 2013;183:1096–112.
Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumors. Nature. 2012;490(7418):61–70.
Mangini NS, Wesolowski R, Ramaswamy B, Lustberg MB, Berger MJ. Palbociclib: a novel cyclin-dependent kinase inhibitor for hormone receptor-positive advanced breast cancer. Ann Pharmacother. 2015;49(11):1252–60.
Finn RS, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11:R77.
Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25–35.
Finn R. Targeting CDK 4/6 in breast cancer [presentation]. San Antonio Breast Cancer Symposium, San Antonio, Texas, Dec 8–12, 2015.
Turner NC, Ro J, Andre F, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373:209–19.
Shapiro G. Beyond CDK 4/6: Targeting additional cell cycle and transcriptional CDKs in breast cancer [presentation]. San Antonio Breast Cancer Symposium, San Antonio, Texas, Dec 8–12, 2015.
Kumar SK, LaPlant B, Chng WJ, et al. Mayo Phase 2 Consortium. Dinaciclib, a novel CDK inhibitor, demonstrates encouraging single-agent activity in patients with relapsed multiple myeloma. Blood. 2015;125(3):443–8.
Eccles SA, Welch DR. Metastasis: recent discoveries and novel treatment strategies. Lancet. 2007;369(9574):1742–57.
Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2(8):563–72.
Uhr JW, Pantel K. Controversies in clinical cancer dormancy. Proc Natl Acad Sci U S A. 2011;108(30):12396–400.
Kang Y, Pantel K. Tumor cell dissemination: emerging biological insights from animal models and cancer patients. Cancer Cell. 2013;23(5):573–81.
Riethdorf S, Müller V, Zhang L, Rau T, Loibl S, Komor M, et al. Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant geparquattro trial. Clin Cancer Res. 2010;16(9):2634–45.
Alix-Panabières C, Schwarzenbach H, Pantel K. Circulating tumor cells and circulating tumor DNA. Annu Rev Med. 2012;63:199–215.
Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer. 2008;8(5):329–40.
Robertson EG, Baxter G. Tumour seeding following percutaneous needle biopsy: the real story! Clin Radiol. 2011;66:1007–14.
Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–92.
Kallergi G, Papadaki MA, Politaki E, et al. Epithelial to mesenchymal transition markers expressed in circulating tumour cells of early and metastatic breast cancer patients. Breast Cancer Res BCR. 2011;13:R59.
Gradilone A, Raimondi C, Nicolazzo C, et al. Circulating tumour cells lacking cytokeratin in breast cancer: the importance of being mesenchymal. J Cell Mol Med. 2011;15:1066–70.
Alix-Panabières C, Pantel K. Challenges in circulating tumour cell research. Nat Rev Cancer. 2014;14(9):623–31.
Kling J. Beyond counting tumor cells. Nat Biotechnol. 2012;30:578–80.
Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–91.
Zhang L, Riethdorf S, Wu G, et al. Meta-analysis of the prognostic value of circulating tumor cells in breast cancer. Clin Cancer Res. 2012;18:5701–10.
Bidard F-C, Peeters DJ, Fehm T, et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 2014;15:406–14.
Pierga J-Y, Hajage D, Bachelot T, et al. High independent prognostic and predictive value of circulating tumor cells compared with serum tumor markers in a large prospective trial in first-line chemotherapy for metastatic breast cancer patients. Ann Oncol. 2012;23:618–24.
Molloy TJ, Devriese LA, Helgason HH, et al. A multimarker QPCR-based platform for the detection of circulating tumour cells in patients with early-stage breast cancer. Br J Cancer. 2011;104:1913–9.
Ferro P, Franceschini MC, Bacigalupo B, et al. Detection of circulating tumour cells in breast cancer patients using human mammaglobin RTPCR: association with clinical prognostic factors. Anticancer Res. 2010;30:2377–82.
Lobodasch K, Frohlich F, Rengsberger M, et al. Quantification of circulating tumour cells for the monitoring of adjuvant therapy in breast cancer: an increase in cell number at completion of therapy is a predictor of early relapse. Breast. 2007;16:211–8.
Rack B, Schindlbeck C, Juckstock J, et al. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J Natl Cancer Inst. 2014;15:106(5).
Ignatiadis M, Riethdorf S, Bidard FC, et al. International study on inter-reader variability for circulating tumor cells in breast cancer. Breast Cancer Res. 2014;16:R43.
Giuliano M, Giordano A, Jackson S, et al. Circulating tumor cells as prognostic and predictive markers in metastatic breast cancer patients receiving first-line systemic treatment. Breast Cancer Res. 2011;13:R67.
Giordano A, Giuliano M, De Laurentiis M, et al. Circulating tumor cells in immunohistochemical subtypes of metastatic breast cancer: lack of prediction in HER2-positive disease treated with targeted therapy. Ann Oncol. 2012;23:1144–50.
Smerage JB, Barlow WE, Hortobagyi GN, et al. Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. J Clin Oncol. 2014;32:3483–89.
Mego M, De Giorgi U, Dawood S, et al. Characterization of metastatic breast cancer patients with nondetectable circulating tumor cells. Int J Cancer J Int Cancer. 2011;129:417–23.
Raimondi C, Gradilone A, Naso G, et al. Epithelial-mesenchymal transition and stemness features in circulating tumor cells from breast cancer patients. Breast Cancer Res Treat. 2011;130:449–55.
Sieuwerts AM, Kraan J, Bolt J, et al. Anti-epithelial cell adhesion molecule antibodies and the detection of circulating normal-like breast tumor cells. J Natl Cancer Inst. 2009;101:61–6.
Mostert B, Kraan J, Bolt-de Vries J, et al. Detection of circulating tumor cells in breast cancer may improve through enrichment with anti-CD146. Breast Cancer Res Treat. 2011;127:33–41.
Weissenstein U, Schumann A, Reif M, Link S, Toffol-Schmidt UD, Heusser P. Detection of circulating tumor cells in blood of metastatic breast cancer patients using a combination of cytokeratin and EpCAM antibodies. BMC Cancer. 2012;12:206.
Yu M, Bardia A, Aceto N, et al. Cancer therapy. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science. 2014;345:216–20.
Krebs MG, Metcalf RL, Carter L, et al. Molecular analysis of circulating tumour cells: biology and biomarkers. Nat Rev Clin Oncol. 2014;11:129–44.
Markou A, Farkona S, Schiza C, et al. PIK3CA mutational status in circulating tumor cells can change during disease recurrence or progression in patients with breast cancer. Clin Cancer Res. 2014;20:5823–34.
Pestrin M, Salvianti F, Galardi F, et al. Heterogeneity of PIK3CA mutational status at the single cell level in circulating tumor cells from metastatic breast cancer patients. Mol Oncol. 2015;9:749–57.
Wang Y, Waters J, Leung ML, et al. Clonal evolution in breast cancer revealed by single nucleus genome sequencing. Nature. 2014;512:155–60.
Onstenk W, Sieuwerts AM, Weekhout M, et al. Gene expression profiles of circulating tumor cells versus primary tumors in metastatic breast cancer. Cancer Lett. 2015;362:36–44.
Hodgson DR, Wellings R, Orr MC, et al. Circulating tumour-derived predictive biomarkers in oncology. Drug Discov Today. 2010;15:98–101.
Board RE, Wardley AM, Dixon JM, et al. Detection of PIK3CA mutations in circulating free DNA in patients with breast cancer. Breast Cancer Res Treat. 2010;120:461–7.
Silva JM, Gonzalez R, Dominguez G, et al. TP53 gene mutations in plasma DNA of cancer patients. Genes Chromosomes Cancer. 1999;24:160–1.
Mouliere F, Robert B, Arnau Peyrotte E, et al. High fragmentation characterizes tumour-derived circulating DNA. PLoS ONE. 2011;6, e23418.
Dawson S-J, Tsui DWY, Murtaza M, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368:1199–209.
Rothe F, Laes JF, Lambrechts D, et al. Plasma circulating tumor DNA as an alternative to metastatic biopsies for mutational analysis in breast cancer. Ann Oncol. 2014;25:1959–65.
Umetani N, Giuliano AE, Hiramatsu SH, et al. Prediction of breast tumor progression by integrity of free circulating DNA in serum. J Clin Oncol. 2006;24:4270–6.
Murtaza M, Dawson S-J, Tsui DWY, et al. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature. 2013;497:108–12.
Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6(224):224ra24.
Sorensen BS, Mortensen LS, Andersen J, Nexo E. Circulating HER2 DNA after trastuzumab treatment predicts survival and response in breast cancer. Anticancer Res. 2010;30:2463–8.
Olsson E, Winter C, George A, Chen Y, Howlin J, Tang MH, et al. Serial monitoring of circulating tumor DNA in patients with primary breast cancer for detection of occult metastatic disease. EMBO Mol Med. 2015;7(8):1034–47.
Higgins MJ, Jelovac D, Barnathan E, et al. Detection of tumor PIK3CA status in metastatic breast cancer using peripheral blood. Clin Cancer Res. 2012;18:3462–9.
Bechmann T, Andersen RF, Pallisgaard N, et al. Plasma HER2 amplification in cell-free DNA during neoadjuvant chemotherapy in breast cancer. J Cancer Res Clin Oncol. 2013;139:995–1003.
Hashad D, Sorour A, Ghazal A, Talaat I. Free circulating tumor DNA as a diagnostic marker for breast cancer. J Clin Lab Anal. 2012;26:467–72.
Leary R, Sausen M, Kinde I, Papadopoulus N, Carpten JD, Craig D, et al. Detection of chromosomal alterations in the circulation of cancer patients with whole genome sequencing. Sci Transl Med. 2012;4(162):162ra154.
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Davies C, Godwin J, Gray R, Clarke M, Cutter D, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771–84.
Ignatiadis M. Multigene assays for late recurrence of breast cancer. Lancet Oncol. 2013;14:1029–30.
Meng S, Tripathy D, Frenkel EP, et al. Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res Off J Am Assoc Cancer Res. 2004;10:8152–62.
Beaver JA, Jelovac D, Balukrishna S, et al. Detection of cancer DNA in plasma of patients with early-stage breast cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2014;20:2643–50.
Shaw JA, Page K, Blighe K, et al. Genomic analysis of circulating cell-free DNA infers breast cancer dormancy. Genome Res. 2012;22:220–31.
Paoletti C, Muniz MC, Thomas DG, et al. Development of circulating tumor cell-endocrine therapy index in patients with hormone receptor positive breast cancer. Clin Cancer Res. 2015;21:2487–98.
Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population based study from the California cancer Registry-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109(9):1721–8.
Schneider BP, Miller KD. Angiogenesis of breast cancer. J Clin Oncol. 2005;23:1782–90.
Saal LH, Holm K, Maurer M, et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2 are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 2005;65:2554–9.
Tryfonopoulos D, Walsh S, Collins DM, et al. Src: a potential target for the treatment of triple-negative breast cancer. Ann Oncol. 2011;22:2234–40.
Dantzer F, de La Rubia G, Ménissier-De Murcia J, et al. Base excision repair is impaired in mammalian cells lacking poly(ADPribose) polymerase-1. Biochemistry. 2000;39:7559–69.
Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121(7):2750–67.
Balko J, Giltriane JM, Wang K, et al. Molecular profiling of the residual disease of triple-negative breast cancer after neoadjuvant chemotherapy identifies actionable therapeutic targets. Cancer Discov. 2014;4:232–45.
A randomized Phase II trial comparing therapy based on tumor molecular profiling versus conventional therapy in patients with refractory cancer (SHIVA). https://clinicaltrials.gov/ct2/show/NCT01771458.
NCI-MPACT: molecular profiling-based assignment of cancer therapy for patients with advanced solid tumors. https://clinicaltrials.gov/ct2/show/NCT01827384.
Molecular profiling in tissue samples from patients with cancer who are exceptional responders to treatment. https://clinicaltrials.gov/ct2/show/NCT02243592.
Vazquez A, Bond EE, Levine AJ, Bond GL. The genetics of the p53 pathway, apoptosis and cancer therapy. Nat Rev Drug Discov. 2008;7(12):979–87.
Khoo KH, Hoe KK, Verma CS, Lane DP. Drugging the p53 pathway: understanding the route to clinical efficacy. Nat Rev Drug Discov. 2014;13(3):217–36.
Tutt A, Robson M, Garber JE, et al. Oral poly (ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376(9737):235–44.
Juric D, Infante J, Krop I et al. Evaluation of tolerability and anti-tumor activity of GDC-0032, a PI3K inhibitor with enhanced activity against PIK3CA mutant tumors, administered to patients with advanced solid tumors. Presented at: European Society of Clinical Oncology Congress. Amsterdam, The Netherlands, 27 September–1 October 2013.
Mayer IA, Abramson V, Lehmann B, et al. New strategies for triple negative breast cancer—deciphering the heterogeneity. Clin Cancer Res. 2014;20:782–90.
Rugo HS, Olopade O, DeMichele A, van’t Veer L, et al. Velaparib/carboplatin plus standard neoadjuvant therapy for high-risk breast cancer: first efficacy results from the I-SPY2 Trial. Proceedings of 31st Annual CTRC-AACR San Antonio Breast Cancer Symposium, Dec 10–14, 2008, SA, TX. Philadelphia, PA: American Association for Cancer Research; [Abstract S5–02].
Moore O, Foote F. The relatively favourable prognosis of medullary carcinoma of the breast. Cancer. 1949;2:635–4.
Hamlin I. Possible host resistance in carcinoma of the breast: a histological study. Br J Cancer. 1968;22(3):383–401.
Rody A, Karn T, Liedtke C, Puztai L, et al. A clinically relevant gene signature in triple negative and basal-like breast cancer. Breast Cancer Res. 2011;13:R97.
Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenco F, et al. Prognostic and predictive value of tumour infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin based chemotherapy: BIG 02–98. J Clin Oncol. 2013;31:860–7.
Telli M. PrECOG 0105: final efficacy analysis from a phase II study of gemcitabine and carboplatin plus iniparib (BSI-201) as neoadjuvant therapy for triple negative and BRCA1/2 mutation associated breast cancer. Proceedings of the 49th Annual Meeting of the American Society of Clinical Oncology (ASCO). Chicago IL: May 31-June 4, 2013; [Abstract 1003].
von Minckwitz Gunter. Geparsixto: addition of carboplatin is beneficial in neoadjuvant treatment of triple negative breast cancer. Proceedings of the 49th Annual Meeting of the American Society of Clinical Oncology (ASCO), Chicago IL: May 31-June 4, 2013;[Abstract 1003].
Basu G, Ghazalpour A, Gatalica Z, et al. Expression of novel Immunotherapy targets in triple negative breast cancer. Proceedings of the 50th Annual Meeting of the American Society of Clinical Oncology (ASCO). Chicago IL: May 31-June 3, 2013; [Abstract 1001].
Mazel M, Jacot W, Alix-Panabières C, et al. Frequent expression of PD-L1 on circulating breast cancer cells. Mol Oncol. 2015;9(9):1773–82.
Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–26.
Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor positive breast cancer. J Clin Oncol. 2006;24:3726–34.
Stephens PJ, Tarpey PS, Davies H, et al. The landscape of cancer genes and mutational processes in breast cancer. Nature. 2012;486:400–4.
Shah SP, Roth A, Goya R, et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 2012;486:395–99.
Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25:1329–33.
Byrski T, Huzarski T, Dent R, et al. Response to neoadjuvant therapy with cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res Treat. 2009;115:359–63.
Abkevich V, Timms KM, Hennessy BT, et al. Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer. Br J Cancer. 2012;107:1776–82.
Birkbak NJ, Wang ZC, Kim JY, et al. Telomeric allelic imbalance indicates defective DNA repair and sensitivity to DNA damaging agents. Cancer Discov. 2012;2:366–75.
Popova T, Manie E, Rieunier G, et al. Ploidy and large-scale genomic instability consistently identify basal-like breast carcinomas with BRCA1/2 inactivation. Cancer Res. 2012;72:5454–62.
Telli M, Jensen K, Abkevich V et al. Homologous recombination deficiency (HRD) score predicts pathologic response following neoadjuvant platinum-based therapy in triple-negative and BRCA1/2 mutation-associated breast cancer (BC). San Antonio Breast Cancer Symposium; 2012, San Antonio.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Hazem I. Assi, Rita E. Assi, and Nagi S. El Saghir declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Biomarkers
Rights and permissions
About this article
Cite this article
Assi, H.I., Assi, R.E. & El Saghir, N.S. Emerging Biomarkers of the Future: Changing Clinical Practice for 2020. Curr Breast Cancer Rep 8, 60–72 (2016). https://doi.org/10.1007/s12609-016-0214-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12609-016-0214-7