Abstract
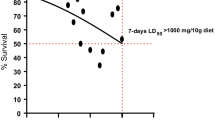
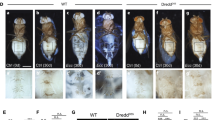
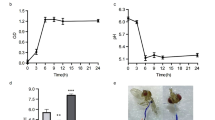
The increasing mortality due to hypertension and hypercholesterolemia is directly linked with type-2 diabetes. This shows the lethality of the disease. Reports suggest that the prebiotics along with probiotics help in lowering the effects of type-2 diabetes. Prebiotic like inulin is best known for its anti-diabetic effect. The current study utilizes jicama extract as prebiotic source of inulin along with the bacterial strains with probiotic properties (Lactiplantibacillus plantarum and Enterococcus faecium) for treating type-2 diabetes in high-fat diet-induced Drosophila melanogaster model. The high-fat diet-induced Drosophila showed deposition of lipid droplets and formation of micronuclei in the gut. The larva and adult treated with probiotics and synbiotic (probiotic + prebiotic- inulin) comparatively reduced the lipid deposition and micronuclei number in the gut. The increased amount of triglyceride in the whole body of the fatty larva and adult indicated the onset of diabetes. The overexpression of insulin-like genes (Dilp 2) and (Dilp 5) confirmed the insulin resistance, whereas the expression was reduced in the larva and adult supplemented with probiotics and synbiotic. The reactive oxygen species level was reduced with the supplementation of probiotics. The weight, larva size, crawling speed and climbing were also altered in high-fat diet-induced Drosophila melanogaster. The study confirmed the effects of probiotics and synbiotic in successfully lowering diabetes in Drosophila. The study also proved the anti-diabetic potential of the probiotics. Further, it was also confirmed that the probiotics work better in the presence of prebiotic.
Graphical Abstract












Similar content being viewed by others
Data Transparency
All data and material regarding this work are completely transparent.
References
Mohan V et al (2021) Efficacy of a combination of metformin and vildagliptin in comparison to metformin alone in type 2 diabetes mellitus: a multicentre, retrospective, real-world evidence study. Diabetes Metab Syndr Obes 14:2925–2933. https://doi.org/10.2147/DMSO.S315227
Bekiari E et al (2016) Systematic review and meta-analysis of vildagliptin for treatment of type 2 diabetes. Endocrine 52(3):458–480. https://doi.org/10.1007/s12020-015-0841-1
Thrasher J (2017) Pharmacologic management of type 2 diabetes mellitus: available therapies. Am J Cardiol 120(1S):S4–S16. https://doi.org/10.1016/j.amjcard.2017.05.009
Naderpoor N et al (2019) Faecal microbiota are related to insulin sensitivity and secretion in overweight or obese adults. J Clin Med 8(4):452. https://doi.org/10.3390/jcm8040452
Yoo JY, Kim SS (2016) Probiotics and prebiotics: present status and future perspectives on metabolic disorders. Nutrients 8(3):173. https://doi.org/10.3390/nu8030173
Marchesi JR et al (2016) The gut microbiota and host health: a new clinical frontier. Gut 65(2):330–339. https://doi.org/10.1136/gutjnl-2015-309990
Mohamadshahi M et al (2014) Effects of probiotic yogurt consumption on inflammatory biomarkers in patients with type 2 diabetes. Bioimpacts 4(2):83–88. https://doi.org/10.5681/bi.2014.007
Blandino G et al (2016) Impact of gut microbiota on diabetes mellitus. Diabetes Metab 42(5):303–315. https://doi.org/10.1016/j.diabet.2016.04.004
Panwar H et al (2013) Probiotics as potential biotherapeutics in the management of type 2 diabetes–prospects and perspectives. Diabetes Metab Res Rev 29(2):103–112. https://doi.org/10.1002/dmrr.2376
Lin SS et al (2021) Mucosal immunity-mediated modulation of the gut microbiome by oral delivery of probiotics into Peyer’s patches. Sci Adv 7(20):eabf0677. https://doi.org/10.1126/sciadv.abf0677
Samah S et al (2016) Probiotics for the management of type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Res Clin Pract 118:172–182. https://doi.org/10.1016/j.diabres.2016.06.014
Kim SW et al (2013) Lactobacillus rhamnosus GG improves insulin sensitivity and reduces adiposity in high-fat diet-fed mice through enhancement of adiponectin production. Biochem Biophys Res Commun 431(2):258–263. https://doi.org/10.1016/j.bbrc.2012.12.121
Pei R et al (2017) Evidence for the effects of yogurt on gut health and obesity. Crit Rev Food Sci Nutr 57(8):1569–1583. https://doi.org/10.1080/10408398.2014.883356
Davani-Davari D et al (2019) Prebiotics: definition, types, sources, mechanisms, and clinical applications. Foods 8(3):92. https://doi.org/10.3390/foods8030092
Ashaolu TJ, Ashaolu JO, Adeyeye SAO (2021) Fermentation of prebiotics by human colonic microbiota in vitro and short-chain fatty acids production: a critical review. J Appl Microbiol 130(3):677–687. https://doi.org/10.1111/jam.14843
Tang R, Li L (2021) Modulation of short-chain fatty acids as potential therapy method for type 2 diabetes mellitus. Can J Infect Dis Med Microbiol. https://doi.org/10.1155/2021/6632266
Puddu A et al (2014) Evidence for the gut microbiota short-chain fatty acids as key pathophysiological molecules improving diabetes. Mediat Inflamm. https://doi.org/10.1155/2014/162021
Kumar J, Rani K, Datt C (2020) Molecular link between dietary fibre, gut microbiota and health. Mol Biol Rep 47(8):6229–6237. https://doi.org/10.1007/s11033-020-05611-3
Nogacka AM et al (2020) In vitro evaluation of different prebiotics on the modulation of gut microbiota composition and function in morbid obese and normal-weight subjects. Int J Mol Sci 21(3):906. https://doi.org/10.3390/ijms21030906
Li K et al (2019) Dietary inulin alleviates diverse stages of type 2 diabetes mellitus via anti-inflammation and modulating gut microbiota in db/db mice. Food Funct 10(4):1915–1927. https://doi.org/10.1039/c8fo02265h
Dong Y et al (2019) The effect of Inulin on lifespan, related gene expression and gut microbiota in InRp5545/TM3 mutant Drosophila melanogaster: A preliminary study. Nutrients 11(3):636. https://doi.org/10.3390/nu11030636
Kobyliak N et al (2018) Effect of alive probiotic on insulin resistance in type 2 diabetes patients: Randomized clinical trial. Diabetes Metab Syndr 12(5):617–624. https://doi.org/10.1016/j.dsx.2018.04.015
Yao K et al (2017) Effect of probiotics on glucose and lipid metabolism in type 2 diabetes mellitus: a meta-analysis of 12 randomized controlled trials. Med Sci Monit 23:3044–3053. https://doi.org/10.12659/msm.902600
Chikkerur J et al (2020) Production of short chain fructo-oligosaccharides from inulin of chicory root using fungal endoinulinase. Appl Biochem Biotechnol 191(2):695–715. https://doi.org/10.1007/s12010-019-03215-7
Stökle K, Jung D, Kruse A (2020) Acid-assisted extraction and hydrolysis of inulin from chicory roots to obtain fructose-enriched extracts. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-020-01108-y
Palanker Musselman L et al (2011) A high-sugar diet produces obesity and insulin resistance in wild-type Drosophila. Dis Model Mech 4(6):842–849. https://doi.org/10.1242/dmm.007948
Praveen MA et al (2019) Dietary fiber from Indian edible seaweeds and its in-vitro prebiotic effect on the gut microbiota. Food Hydrocoll 96:343–353. https://doi.org/10.1016/j.foodhyd.2019.05.031
Shweta T et al (2015) Microwave-assisted extraction of inulin from chicory roots using response surface methodology. J Nutr Food Sci 5(1):1–7
Petkova N, Sherova G, Denev P (2018) Characterization of inulin from dahlia tubers isolated by microwave and ultrasound-assisted extractions. Int Food Res J 25(5)
Sabat D et al (2015) A protocol to generate germ free Drosophila for microbial interaction studies. Adv. Tech. Biol. Med. S 1:2379–1764. https://doi.org/10.4172/2379-1764.S1-001
Nayak N, Mishra M (2021) High fat diet induced abnormalities in metabolism, growth, behavior, and circadian clock in Drosophila melanogaster. Life Sci 281:119758. https://doi.org/10.1016/j.lfs.2021.119758
Westfall S, Lomis N, Prakash S (2018) A polyphenol-rich prebiotic in combination with a novel probiotic formulation alleviates markers of obesity and diabetes in Drosophila. J Funct Foods 48:374–386. https://doi.org/10.1016/j.jff.2018.07.012
Priyadarsini S et al (2019) An infection of Enterobacter ludwigii affects development and causes age-dependent neurodegeneration in Drosophila melanogaster. Invert Neurosci 19(4):13. https://doi.org/10.1007/s10158-019-0233-y
Dhar G et al (2020) Various behavioural assays to detect the neuronal abnormality in flies. In fundamental approaches to screen abnormalities in Drosophila. Springer, p 223–251. https://doi.org/10.1007/978-1-4939-9756-5_18
Priyadarsini S, Mukherjee S, Mishra M (2020) Methodology to Detect the Abnormality of Drosophila Gut by Various Staining Techniques. In Fundamental Approaches to Screen Abnormalities in Drosophila. Springer, p 51–64. https://doi.org/10.1007/978-1-4939-9756-5_5
Liu J et al (2012) Synphilin-1 alters metabolic homeostasis in a novel Drosophila obesity model. Int J Obes (Lond) 36(12):1529–1536. https://doi.org/10.1038/ijo.2012.111
Liu Z et al (2008) A Drosophila model for LRRK2-linked parkinsonism. Proc Natl Acad Sci U S A 105(7):2693–2698. https://doi.org/10.1073/pnas.0708452105
Nichols CD, Becnel J, Pandey UB (2012) Methods to assay Drosophila behavior. J Vis Exp 61:e3795. https://doi.org/10.3791/3795
Jovanovic B, Cvetkovic VJ, Mitrovic TLJ (2016) Effects of human food grade titanium dioxide nanoparticle dietary exposure on Drosophila melanogaster survival, fecundity, pupation and expression of antioxidant genes. Chemosphere 144:43–49. https://doi.org/10.1016/j.chemosphere.2015.08.054
Nayak N, Mishra M (2020) Estimation of Oxidative Stress and Survivorship in Drosophila. In Fundamental Approaches to Screen Abnormalities in Drosophila. Springer, p 123–134. https://doi.org/10.1007/978-1-4939-9756-5_11
Nayak N, Mishra M (2019) Simple techniques to study multifaceted diabesity in the fly model. Toxicol Mech Methods 29(8):549–560. https://doi.org/10.1080/15376516.2019.1634171
Mukherjee S, Mishra M (2020) Biochemical Estimation to Detect the Metabolic Pathways of Drosophila. In Fundamental Approaches to Screen Abnormalities in Drosophila. Springer, p 135–149. https://doi.org/10.1007/978-1-4939-9756-5_12
Haddad MA (2017) Viability of probiotic bacteria during refrigerated storage of commercial probiotic fermented dairy products marketed in Jordan. J Food Res 6(2):75–81. https://doi.org/10.5539/jfr.v6n2p75
Pappus SA et al (2017) A toxicity assessment of hydroxyapatite nanoparticles on development and behaviour of Drosophila melanogaster. J Nanopart Res 19(4):136. https://doi.org/10.1007/S11051-017-3824-8
Kahn SE, Hull RL, Utzschneider KM (2006) Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444(7121):840–846. https://doi.org/10.1038/nature05482
DeGruttola AK et al (2016) Current understanding of dysbiosis in disease in human and animal models. Inflamm Bowel Dis 22(5):1137–1150. https://doi.org/10.1097/MIB.0000000000000750
Delzenne NM et al (2011) Targeting gut microbiota in obesity: effects of prebiotics and probiotics. Nat Rev Endocrinol 7(11):639–646. https://doi.org/10.1038/nrendo.2011.126
Feng T, Wang J (2020) Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: a systematic review. Gut Microbes 12(1):1801944. https://doi.org/10.1080/19490976.2020.1801944
Musselman LP et al (2018) A complex relationship between immunity and metabolism in Drosophila diet-induced insulin resistance. Mol Cell Biol 38(2):e00259-e317. https://doi.org/10.1128/MCB.00259-17
Bäckhed F et al (2004) The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci 101(44):15718–15723. https://doi.org/10.1073/pnas.0407076101
Vrieze A et al (2012) Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143(4):913-916.e7. https://doi.org/10.1053/j.gastro.2012.06.031
He FF, Li YM (2020) Role of gut microbiota in the development of insulin resistance and the mechanism underlying polycystic ovary syndrome: a review. J Ovarian Res 13(1):73. https://doi.org/10.1186/s13048-020-00670-3
Pasco MY, Leopold P (2012) High sugar-induced insulin resistance in Drosophila relies on the lipocalin Neural Lazarillo. PLoS ONE 7(5):e36583. https://doi.org/10.1371/journal.pone.0036583
Lourido F et al (2021) Domeless receptor loss in fat body tissue reverts insulin resistance induced by a high-sugar diet in Drosophila melanogaster. Sci Rep 11(1):3263. https://doi.org/10.1038/s41598-021-82944-4
Katsube H et al (2019) Endoplasmic reticulum stress-induced cellular dysfunction and cell death in insulin-producing cells results in diabetes-like phenotypes in Drosophila. Biol Open 8(12):bio046524. https://doi.org/10.1242/bio.046524
Zhang Y, Xi Y (2015) Fat body development and its function in energy storage and nutrient sensing in Drosophila melanogaster. J Tissue Sci Eng 6(1):1
Siddiqui L et al (2020) Assessing the potential of lignin nanoparticles as drug carrier: Synthesis, cytotoxicity and genotoxicity studies. Int J Biol Macromol 152:786–802. https://doi.org/10.1016/j.ijbiomac.2020.02.311
Farhad N, Shahsavari S (2020) Probiotics and their effects in reduction hyperlipidemia. Plant Biotechnol Persa 2(2):21–23. https://doi.org/10.52547/pbp.2.2.21
Cavalcante RGS et al (2019) The probiotic Lactobacillus fermentum 296 attenuates cardiometabolic disorders in high fat diet-treated rats. Nutr Metab Cardiovasc Dis 29(12):1408–1417. https://doi.org/10.1016/j.numecd.2019.08.003
Schiesari L et al (2016) The Insulin-Like Proteins dILPs-2/5 Determine Diapause Inducibility in Drosophila. PLoS ONE 11(9):e0163680. https://doi.org/10.1371/journal.pone.0163680
Moghadam NN et al (2015) The role of storage lipids in the relation between fecundity, locomotor activity, and lifespan of Drosophila melanogaster longevity-selected and control lines. PLoS ONE 10(6):e0130334. https://doi.org/10.1371/journal.pone.0130334
Ramachandran A (2014) Know the signs and symptoms of diabetes. Indian J Med Res 140(5):579–581
Mallick T et al (2020) Carbazole analog anchored fluorescent silica nanoparticle showing enhanced biocompatibility and selective sensing ability towards biomacromolecule. Dyes Pigm 173:107994. https://doi.org/10.1016/J.Dyepig.2019.107994
Lau AWY et al (2021) The chemistry of gut microbiome in health and diseases. Progress in Microbes & Molecular Biology 4(1). https://doi.org/10.36877/pmmb.a0000175
Acknowledgements
The authors are thankful to the Science & Engineering Research Board (SERB) for financial support and National Institute of Technology (NIT), Rourkela, for providing the research facilities. The study was also supported by funding from Department of Biotechnology (DBT) under grant no. BT/PR21857/NNT/28/1238/2017 and SERB under grant no. EMR/2017/003054, received by Dr. Monalisa Mishra. Dr. Moumita Sahoo is highly acknowledged for providing the isolated probiotic strains for carrying out the research work.
Funding
AB is thankful to the Science & Engineering Research Board (SERB), (File No. IMP/2018/002120/EC), Government of India, for the financial support. NN is thankful to DST, INSPIRE for the financial support and SM is thankful to the Ministry of Human Resource and Development (MHRD). The work was also supported by funding from Department of Biotechnology (DBT) under grant no. BT/PR21857/NNT/28/1238/2017 and SERB under grant no. EMR/2017/003054, received by Dr. Monalisa Mishra.
Author information
Authors and Affiliations
Contributions
Amrita Bhanja was involved in methodology, data curation, formal analysis, investigation, manuscript writing; Nibedita Nayak helped in data curation, investigation, formal analysis; Sumit Mukherjee contributed to data curation, investigation, formal analysis; Parag Prakash Sutar was involved in conceptualization, methodology, writing—original draft, writing—review and editing, funding acquisition; Monalisa Mishra helped in conceptualization, methodology, writing—original draft, writing—review and editing, funding acquisition, resources, supervision.
Corresponding author
Ethics declarations
Ethics Approval
None.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bhanja, A., Nayak, N., Mukherjee, S. et al. Treating the Onset of Diabetes Using Probiotics Along with Prebiotic from Pachyrhizus erosus in High-Fat Diet Fed Drosophila melanogaster. Probiotics & Antimicro. Prot. 14, 884–903 (2022). https://doi.org/10.1007/s12602-022-09962-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-022-09962-0