Abstract
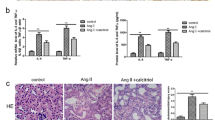
To investigate the effects of κ-OR agonist on hyperuricemia rats and injured endothelial function, as well as the underlying mechanism. A hyperuricemia model was established on rats. The endothelial protective effects of U50,488H were evaluated and compared to the controlled groups. The protein levels of eNOS, p-eNOS, Akt, and p-Akt were determined using western blot analysis. ELISA was employed to measure the expression of soluble ET-1, ICAM-1, TNF-α, and NO in cell supernatants and rat serum samples. Cell migration and the artery tension were determined by in vitro functional assays. The suppressed production of ET-1, ICAM-1, and NO in the hyperuricemia rats was promoted by the treatment of U50,488H, which was reversed by the co-administration of nor-BNI. P-eNOS/eNOS and p-Akt/Akt were up-regulated by the incubation of serum from hyperuricemia rats, which was down-regulated by the introduction of U50,488H. The vascular tension of vessels incubated with U50,488H was higher than the baseline in the presence of ACh, which was lower than baseline in the presence of SNAP. U50,488H significantly promoted the release of ET-1, ICAM-1, and NO, and inhibited the release of TNF-α from endothelial cells and the migration ability of neutrophils in the presence of hyperuricemia rat serum, which were reversed by the co-incubation with nor-BNI, Akt inhibitor or L-NAME. U50,488H protected the endothelial function impaired by hyperuricemia through regulating the Akt/eNOS signal pathway.





Similar content being viewed by others
References
Borghi C, Palazzuoli A, Landolfo M, Cosentino E (2020) Hyperuricemia: a novel old disorder-relationship and potential mechanisms in heart failure. Heart Fail 25:43–51
Kuriyama S (2020) Dotinurad: a novel selective urate reabsorption inhibitor as a future therapeutic option for hyperuricemia. Clin Exp Nephrol 24:6–7
Watanabe S, Kang DH, Feng L, Nakagawa T et al (2002) Uric acid, hominoid evolution, and the pathogenesis of salt-sensitivity. Hypertension 40:355–360
Mazzali M, Hughes J, Kim YG, Jefferson JA et al (2001) Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension 38:1101–1106
Erdogan D, Gullu H, Caliskan M et al (2005) Relationship of serum uric acid to measures of endothelial function and atherosclerosis in healthy adults. Int J Clin Pract 59:1276–1282
Kanabrocki EL, Third JL, Ryan MD et al (2000) Circadian relationship of serum uric acid and nitric oxide. JAMA 283:2240–2241
Kato M, Hisatome I, Tomikura Y et al (2005) Status of endothelial dependent vasodilation in patients with hyperuricemia. Am J Cardiol 96:1576–1578
Zou H, Wang H, Liu T et al (2017) Protective role of alpha-lipoic acid in hyperuricemia-induced endothelial dysfunction. Exp Ther Med 13:3047–3054
Zhen H, Gui F (2017) The role of hyperuricemia on vascular endothelium dysfunction. Biomed Rep 7:325–330
Liu S, Yuan Y, Zhou Y et al (2017) Phloretin attenuates hyperuricemia-induced endothelial dysfunction through co-inhibiting inflammation and GLUT9-mediated uric acid uptake. J Cell Mol Med 21(10):2553–2562
Kondratiuk VE, Tarasenko OM, Karmazina OM et al (2020) Impact of the synbiotics and urate-lowering therapy on gut microbiota and cytokine profile in patients with chronic gouty arthritis. J Med Life 13(4):490–498
Reid G, Abrahamsson T, Bailey M et al (2017) How do probiotics and prebiotics function at distant sites? Benef Microbes 8:521–533
Gibson GR, Hutkins R, Sanders ME et al (2017) Expert consensus document: the international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol 14(8):491–502
Salminen S, Collado MC, Endo A et al (2021) The international scientific association of probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol 18(9):649–667
Peng P, Huang LY, Li J et al (2009) Distribution of kappa-opioid receptor in the pulmonary artery and its changes during hypoxia. Anat Rec (Hoboken) 292:1062–1067
Gross ER, Hsu AK, Gross GJ (2006) The JAK/STAT pathway is essential for opioid-induced cardioprotection: JAK2 as a mediator of STAT3, Akt, and GSK-3 beta. Am J Physiol Heart Circ Physiol 291:H827-834
Fryer RM, Pratt PF, Hsu AK et al (2001) Differential activation of extracellular signal regulated kinase isoforms in preconditioning and opioid-induced cardioprotection. J Pharmacol Exp Ther 296:642–649
Fryer RM, Wang Y, Hsu AK et al (2001) Dependence of delta1-opioid receptor-induced cardioprotection on a tyrosine kinase-dependent but not a Src-dependent pathway. J Pharmacol Exp Ther 299:477–482
Shi XL, Qiao M, Wu MJ et al (2019) Overview of animal models of gout and hyperuricemia and metabolic pathway analysis. Tianjin J Tradit Chin Med 36:511–515
Ikenaga T, Noguchi H, Kakumoto K et al (2020) Effect of phytic acid on postprandial serum uric acid level in healthy volunteers: a randomized, double-blind, crossover study. Nucleosides Nucleotides Nucleic Acids 39:504–517
Scott GS, Hooper DC (2001) The role of uric acid in protection against peroxynitrite-mediated pathology. Med Hypotheses 56:95–100
Becker BF, Reinholz N, Leipert B et al (1991) Role of uric acid as an endogenous radical scavenger and antioxidant. Chest 100:176S-181S
Skinner KA, White CR, Patel R et al (1998) Nitrosation of uric acid by peroxynitrite. Formation of a vasoactive nitric oxide donor. J Biol Chem 273:24491–24497
Hink HU, Santanam N, Dikalov S et al (2002) Peroxidase properties of extracellular superoxide dismutase: role of uric acid in modulating in vivo activity. Arterioscler Thromb Vasc Biol 22:1402–1408
Hong Q, Qi K, Feng Z et al (2012) Hyperuricemia induces endothelial dysfunction via mitochondrial Na+/Ca2+ exchanger-mediated mitochondrial calcium overload. Cell Calcium 51:402–410
Kang DH, Park SK, Lee IK et al (2005) Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol 16:3553–3562
Zharikov S, Krotova K, Hu H et al (2008) ‘Uric acid decreases NO production and increases arginase activity in cultured pulmonary artery endothelial cells. Am J Physiol Cell Physiol 295:C1183-1190
Lu J, Sun M, Wu X et al (2019) Urate-lowering therapy alleviates atherosclerosis inflammatory response factors and neointimal lesions in a mouse model of induced carotid atherosclerosis. FEBS J 286:1346–1359
MacRae K, Connolly K, Vella R et al (2019) Epicatechin’s cardiovascular protective effects are mediated via opioid receptors and nitric oxide. Eur J Nutr 58:515–527
Naryzhnaya NV, Khaliulin I, Lishmanov YB et al (2019) Participation of opioid receptors in the cytoprotective effect of chronic normobaric hypoxia. Physiol Res 68:245–253
Habas K, Shang L (2018) Alterations in intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) in human endothelial cells. Tissue Cell 54:139–143
Chen L, Qin L, Liu X et al (2019) CTRP3 alleviates Ox-LDL-induced inflammatory response and endothelial dysfunction in mouse aortic endothelial cells by activating the PI3K/Akt/eNOS pathway. Inflammation 42:1350–1359
Mahmoud AM, Wilkinson FL, McCarthy EM et al (2017) Endothelial microparticles prevent lipid-induced endothelial damage via Akt/eNOS signaling and reduced oxidative stress. FASEB J 31:4636–4648
Chen Z, Oliveira SDS, Zimnicka AM et al (2018) Reciprocal regulation of eNOS and caveolin-1 functions in endothelial cells. Mol Biol Cell 29:1190–1202
Xia W, Yin J, Zhang S et al (2018) ‘Parkin modulates ERRalpha/eNOS signaling pathway in endothelial cells. Cell Physiol Biochem 49:2022–2034
Xu Y, Sui L, Qiu B et al (2019) ANXA4 promotes trophoblast invasion via the PI3K/Akt/eNOS pathway in preeclampsia. Am J Physiol Cell Physiol 316:C481–C491
Maiuolo J, Oppedisano F, Gratteri S et al (2016) Regulation of uric acid metabolism and excretion. Int J Cardiol 213:8–14
Hyndman D, Liu S, Miner JN (2016) Urate handling in the human body. Curr Rheumatol Rep 18:34
Guo Z, Zhang J, Wang Z et al (2016) Intestinal microbiota distinguish gout patients from healthy humans. Sci Rep 6:20602
Bubnov R, Babenko L, Lazarenko L et al (2019) Can tailored nanoceria act as a prebiotic? Report on improved lipid profile and gut microbiota in obese mice. EPMA J 10(4):317–335
Reid G, Abrahamsson T, Bailey M et al (2017) How do probiotics and prebiotics function at distant sites? Benef Microbes 8(4):521–533
Bubnov R, Polivka J Jr, Zubor P et al (2017) Pre-metastatic niches” in breast cancer: are they created by or prior to the tumour onset? “Flammer Syndrome” relevance to address the question. EPMA J 8(2):141–157
Wang F, Meng J, Zhang L et al (2018) Morphine induces changes in the gut microbiome and metabolome in a morphine dependence model. Sci Rep 8(1):3596
Funding
The general fund projects of Sichuan Provincial Department of Education (18ZB0177).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qin Zheng and Qi Wu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zheng, Q., Wu, Q., Yang, H. et al. A κ-OR Agonist Protects the Endothelial Function Impaired by Hyperuricemia Through Regulating the Akt/eNOS Signal Pathway. Probiotics & Antimicro. Prot. 14, 751–759 (2022). https://doi.org/10.1007/s12602-022-09945-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-022-09945-1