Abstract
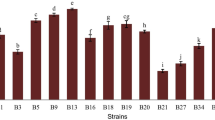
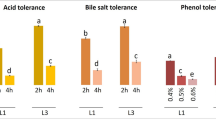
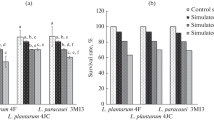
The increased consumers’ interest on the positive role of food in wellbeing and health underscores the need to determine new probiotic microorganisms. Triggered by the fact that artisanal food products can be a valuable source of novel probiotic strains, 106 lactic acid bacteria, all isolated from traditional Greek dairy products, namely Feta, Kasseri, Xynotyri, Graviera, Formaela, Galotyri, and Kefalotyri cheeses as well as yogurt and milk, were studied for probiotic properties. Based on their survival at pH 2.5 and their stability in the presence of bile salts, 20 strains were selected for further analysis. These strains exhibited diverse susceptibility to commonly used antibiotics, while none was hemolytic. Seven out of the 20 strains produced functional bile salt hydrolases in vitro. The only antimicrobial activity detected of Streptococcus thermophilus ACA-DC 26 against the oral pathogen Streptococcus mutans LMG 14558T was attributed to compound(s) of proteinaceous nature. Two Lactobacillus plantarum strains, namely ACA-DC 2640 and ACA-DC 4039, displayed the highest adhesion according to a collagen-based microplate assay and by using ΗΤ-29 and Caco-2 cells. Co-cultivation of THP-1 cells with selected strains indicated a tendency for anti-inflammatory modulation by Lactobacillus plantarum ACA-DC 2640 as well as Streptococcus thermophilus ACA-DC 26 and ACA-DC 170, as shown by an increase in IL10 mRNA levels. Moreover, milk cell-free supernatants of Lactobacillus plantarum ACA-DC 2640 and ACA-DC 4039 exhibited strong angiotensin I-converting enzyme inhibition. To conclude, new isolates presenting interesting probiotic features were described and should be further investigated as health-promoting factors.



Similar content being viewed by others
References
Thomas LV (2016) Probiotics—the journey continues. Int J Dairy Technol 69(4):469–480. doi:10.1111/1471-0307.12354
Zoumpopoulou G, Pot B, Tsakalidou E, Papadimitriou K (2017) Dairy probiotics: beyond the role of promoting gut and immune health. Int Dairy J 67:46–60. doi:10.1016/j.idairyj.2016.09.010
Santos TT, Ornellas RMS, Arcucio LB, Oliveira MM, Nicoli JR, Dias CV, Uetanabaro APT, Vinderola G (2016) Characterization of lactobacilli strains derived from cocoa fermentation in the south of Bahia for the development of probiotic cultures. LWT Food Sci Technol 73:259–266. doi:10.1016/j.lwt.2016.06.003
Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods. Off J Eur Union L12:3–18
Italian Ministry of Health (2013) Guidelines on probiotics and prebiotics. Internet document URL http://www.salute.gov.it/imgs/C_17_pubblicazioni_1016_ulterioriallegati_ulterioreallegato_0_alleg.pdf
Papadimitriou K, Zoumpopoulou G, Foligné B, Alexandraki V, Kazou M, Pot B, Tsakalidou E (2015) Discovering probiotic microorganisms: in vitro, in vivo, genetic and omics approaches. Front Microbiol 6:58. doi:10.3389/fmicb.2015.00058
Litopoulou-Tzanetaki E, Tzanetakis N (2011) Microbiological characteristics of Greek traditional cheeses. Small Rumin Res 101(1–3):17–32. doi:10.1016/j.smallrumres.2011.09.022
Zoumpopoulou G, Foligne B, Christodoulou K, Grangette C, Pot B, Tsakalidou E (2008) Lactobacillus fermentum ACA-DC 179 displays probiotic potential in vitro and protects against trinitrobenzene sulfonic acid (TNBS)-induced colitis and Salmonella infection in murine models. Int J Food Microbiol 121(1):18–26. doi:10.1016/j.ijfoodmicro.2007.10.013
Tram G, Korolik V, Day CJ (2013) MBDS solvent: an improved method for assessment of biofilms. Adv Microbiol 3:200–204. doi:10.4236/aim.2013.32030
Siegesmund AM, Konkel ME, Klena JD, Mixter PF (2004) Campylobacter jejuni infection of differentiated THP-1 macrophages results in interleukin 1β release and caspase-1-independent apoptosis. Microbiol 150(3):561–569. doi:10.1099/mic.0.26466-0
Dimozi A, Mavrogonatou E, Sklirou A, Kletsas D (2015) Oxidative stress inhibits the proliferation, induces premature senescence and promotes a catabolic phenotype in human nucleus pulposus intervertebral disc cells. Eur Cell Mater 30:89–102; discussion 103. doi:10.22203/eCM.v030a07
Muguerza B, Ramos M, Sánchez E, Manso MA, Miguel M, Aleixandre A, Delgado MA, Recio I (2006) Antihypertensive activity of milk fermented by Enterococcus faecalis strains isolated from raw milk. Int Dairy J 16(1):61–69. doi:10.1016/j.idairyj.2005.01.001
Quirós A, Ramos M, Muguerza B, Delgado MA, Miguel M, Aleixandre A, Recio I (2007) Identification of novel antihypertensive peptides in milk fermented with Enterococcus faecalis. Int Dairy J 17(1):33–41. doi:10.1016/j.idairyj.2005.12.011
Pan D, Luo Y, Tanokura M (2005) Antihypertensive peptides from skimmed milk hydrolysate digested by cell-free extract of Lactobacillus helveticus JCM1004. Food Chem 91(1):123–129. doi:10.1016/j.foodchem.2004.05.055
EFSA (2012) Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J 10(6):2740-n/a. doi:10.2903/j.efsa.2012.2740
Amund OD (2016) Exploring the relationship between exposure to technological and gastrointestinal stress and probiotic functional properties of lactobacilli and bifidobacteria. Can J Microbiol 62(9):715–725. doi:10.1139/cjm-2016-0186
Devirgiliis C, Barile S, Perozzi G (2011) Antibiotic resistance determinants in the interplay between food and gut microbiota. Genes Nutr 6(3):275–284. doi:10.1007/s12263-011-0226-x
Gueimonde M, Sanchez B, GdLR-G C, Margolles A (2013) Antibiotic resistance in probiotic bacteria. Front Microbiol 4:202. doi:10.3389/fmicb.2013.00202
Salvetti E, Orrù L, Capozzi V, Martina A, Lamontanara A, Keller D, Cash H, Felis GE, Cattiveli L, Torriani S, Spano G (2016) Integrate genome-based assessment of safety for probiotic strains: Bacillus coagulans GBI-30, 6086 as a case study. Appl Microbiol Biotechnol 100(10):4595–4605. doi:10.1007/s00253-016-7416-9
de Moraes GMD, de Abreu LR, do Egito AS, Salles HO, da Silva LMF, Nero LA, Todorov SD, dos Santos KMO (2016) Functional properties of Lactobacillus mucosae strains isolated from Brazilian goat milk. Probiotics Antimicrob Proteins 1–11. doi:10.1007/s12602-016-9244-8
Ouwehand AC, Forssten S, Hibberd AA, Lyra A, Stahl B (2016) Probiotic approach to prevent antibiotic resistance. Ann Med 48(4):246–255. doi:10.3109/07853890.2016.1161232
Ammor MS, Florez AB, Mayo B (2007) Antibiotic resistance in non-enterococcal lactic acid bacteria and bifidobacteria. Food Microbiol 24(6):559–570. doi:10.1016/j.fm.2006.11.001
Bernardeau M, Vernoux JP, Henri-Dubernet S, Guéguen M (2008) Safety assessment of dairy microorganisms: the Lactobacillus genus. Int J Food Microbiol 126(3):278–285. doi:10.1016/j.ijfoodmicro.2007.08.015
Guidone A, Zotta T, Ross RP, Stanton C, Rea MC, Parente E, Ricciardi A (2014) Functional properties of Lactobacillus plantarum strains: a multivariate screening study. LWT Food Sci Technol 56(1):69–76. doi:10.1016/j.lwt.2013.10.036
Nawaz M, Wang J, Zhou A, Ma C, Wu X, Moore JE, Millar BC, Xu J (2011) Characterization and transfer of antibiotic resistance in lactic acid bacteria from fermented food products. Curr Microbiol 62(3):1081–1089. doi:10.1007/s00284-010-9856-2
Hashemi SM, Shahidi F, Mortazavi SA, Milani E, Eshaghi Z (2014) Potentially probiotic Lactobacillus strains from traditional Kurdish cheese. Probiotics Antimicrob Proteins 6(1):22–31. doi:10.1007/s12602-014-9155-5
Chand D, Avinash VS, Yadav Y, Pundle AV, Suresh CG, Ramasamy S (2017) Molecular features of bile salt hydrolases and relevance in human health. Biochim Biophys Acta 1861:2981–2991. doi:10.1016/j.bbagen.2016.09.024
Joyce SA, Shanahan F, Hill C, Gahan CG (2014) Bacterial bile salt hydrolase in host metabolism: potential for influencing gastrointestinal microbe-host crosstalk. Gut Microbes 5(5):669–674. doi:10.4161/19490976.2014.969986
Begley M, Hill C, Gahan CG (2006) Bile salt hydrolase activity in probiotics. Appl Environ Microbiol 72(3):1729–1738. doi:10.1128/aem.72.3.1729-1738.2006
Lv LX, Yan R, Shi HY, Shi D, Fang DQ, Jiang HY, Wu WR, Guo FF, Jiang XW, Gu SL, Chen YB, Yao J, Li LJ (2017) Integrated transcriptomic and proteomic analysis of the bile stress response in probiotic Lactobacillus salivarius LI01. J Proteome 150:216–229. doi:10.1016/j.jprot.2016.08.021
Patel AK, Singhania RR, Pandey A, Chincholkar SB (2010) Probiotic bile salt hydrolase: current developments and perspectives. Appl Biochem Biotechnol 162(1):166–180. doi:10.1007/s12010-009-8738-1
Archer AC, Halami PM (2015) Probiotic attributes of Lactobacillus fermentum isolated from human feces and dairy products. Appl Microbiol Biotechol 99(19):8113–8123. doi:10.1007/s00253-015-6679-x
Vizoso Pinto MG, Franz CM, Schillinger U, Holzapfel WH (2006) Lactobacillus spp. with in vitro probiotic properties from human faeces and traditional fermented products. Int J Food Microbiol 109(3):205–214. doi:10.1016/j.ijfoodmicro.2006.01.029
Miquel S, Beaumont M, Martin R, Langella P, Braesco V, Thomas M (2015) A proposed framework for an appropriate evaluation scheme for microorganisms as novel foods with a health claim in Europe. Microb Cell Factories 14:48. doi:10.1186/s12934-015-0229-1
Reid G, Younes JA, Van der Mei HC, Gloor GB, Knight R, Busscher HJ (2011) Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol 9(1):27–38. doi:10.1038/nrmicro2473
Zoumpopoulou G, Pepelassi E, Papaioannou W, Georgalaki M, Maragkoudakis PA, Tarantilis PA, Polissiou M, Tsakalidou E, Papadimitriou K (2013) Incidence of bacteriocins produced by food-related lactic acid bacteria active towards oral pathogens. Int J Mol Sci 14(3):4640–4654. doi:10.3390/ijms14034640
Bermudez-Brito M, Plaza-Diaz J, Munoz-Quezada S, Gomez-Llorente C, Gil A (2012) Probiotic mechanisms of action. Ann Nutr Metab 61(2):160–174. doi:10.1159/000342079
Sengupta R, Altermann E, Anderson RC, McNabb WC, Moughan PJ, Roy NC (2013) The role of cell surface architecture of lactobacilli in host-microbe interactions in the gastrointestinal tract. Mediat Inflamm 2013:16. doi:10.1155/2013/237921
Fabien JC, Stephanie-Marie D, Adriana Perez C, Benoit F, Gwenael J (2012) Interactions between probiotic dairy propionibacteria and the intestinal epithelium. Curr Immunol Rev 8(3):216–226. doi:10.2174/157339512800671976
Lievin-Le Moal V, Servin AL (2013) Pathogenesis of human enterovirulent bacteria: lessons from cultured, fully differentiated human colon cancer cell lines. Microbiol Mol Biol Rev 77(3):380–439. doi:10.1128/mmbr.00064-12
Gagnon M, Zihler Berner A, Chervet N, Chassard C, Lacroix C (2013) Comparison of the Caco-2, HT-29 and the mucus-secreting HT29-MTX intestinal cell models to investigate Salmonella adhesion and invasion. J Microbiol Methods 94(3):274–279. doi:10.1016/j.mimet.2013.06.027
Wang B, Wei H, Yuan J, Li Q, Li Y, Li N, Li J (2008) Identification of a surface protein from Lactobacillus reuteri JCM1081 that adheres to porcine gastric mucin and human enterocyte-like HT-29 cells. Curr Microbiol 57(1):33–38. doi:10.1007/s00284-008-9148-2
Laparra JM, Sanz Y (2009) Comparison of in vitro models to study bacterial adhesion to the intestinal epithelium. Lett Appl Microbiol 49(6):695–701. doi:10.1111/j.1472-765X.2009.02729.x
Mosser DM, Edwards JP (2008) Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8(12):958–969. doi:10.1038/nri2448
Qin Z (2012) The use of THP-1 cells as a model for mimicking the function and regulation of monocytes and macrophages in the vasculature. Atherosclerosis 221(1):2–11. doi:10.1016/j.atherosclerosis.2011.09.003
Cassetta L, Cassol E, Poli G (2011) Macrophage polarization in health and disease. Sci World J 11:2391–2402. doi:10.1100/2011/213962
Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25(12):677–686. doi:10.1016/j.it.2004.09.015
Lumeng CN, Bodzin JL, Saltiel AR (2007) Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117(1):175–184. doi:10.1172/JCI29881
Kim HJ, Higashimori T, Park SY, Choi H, Dong J, Kim YJ, Noh HL, Cho YR, Cline G, Kim YB, Kim JK (2004) Differential effects of interleukin-6 and -10 on skeletal muscle and liver insulin action in vivo. Diabetes 53(4):1060–1067
Meshkani R, Adeli K (2009) Hepatic insulin resistance, metabolic syndrome and cardiovascular disease. Clin Biochem 42(13–14):1331–1346. doi:10.1016/j.clinbiochem.2009.05.018
Forstermann U, Sessa WC (2012) Nitric oxide synthases: regulation and function. Eur Heart J 33(7):829–837, 837a-837d. doi:10.1093/eurheartj/ehr304
Ricciotti E, FitzGerald GA (2011) Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol 31(5):986–1000. doi:10.1161/ATVBAHA.110.207449
Fitzgerald RJ, Murray BA (2006) Bioactive peptides and lactic fermentations. Int J Dairy Technol 59(2):118–125. doi:10.1111/j.1471-0307.2006.00250.x
Masuyer G, Schwager SL, Sturrock ED, Isaac RE, Acharya KR (2012) Molecular recognition and regulation of human angiotensin-I converting enzyme (ACE) activity by natural inhibitory peptides. Sci Rep 2:717. doi:10.1038/srep00717
Rutella GS, Tagliazucchi D, Solieri L (2016) Survival and bioactivities of selected probiotic lactobacilli in yogurt fermentation and cold storage: new insights for developing a bi-functional dairy food. Food Microbiol 60:54–61. doi:10.1016/j.fm.2016.06.017
Nurminen ML, Sipola M, Kaarto H, Pihlanto-Leppala A, Piilola K, Korpela R, Tossavainen O, Korhonen H, Vapaatalo H (2000) Alpha-lactorphin lowers blood pressure measured by radiotelemetry in normotensive and spontaneously hypertensive rats. Life Sci 66(16):1535–1543. doi:10.1016/S0024-3205(00)00471-9
Seppo L, Jauhiainen T, Poussa T, Korpela R (2003) A fermented milk high in bioactive peptides has a blood pressure-lowering effect in hypertensive subjects. Am J Clin Nutr 77(2):326–330
Acknowledgements
This work was co-financed by the European Regional Development Fund (ERDF) of the EU and by the Greek National Resources under the Operational Program Competitiveness and Entrepreneurship (OPCE II), Action “COOPERATION 2011”, Partnerships of Production and Research Institutions in Focused Research and Technology Sectors—Project 11SYN_2_571_ProbioDairyMeat.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 171 kb)
Rights and permissions
About this article
Cite this article
Zoumpopoulou, G., Tzouvanou, A., Mavrogonatou, E. et al. Probiotic Features of Lactic Acid Bacteria Isolated from a Diverse Pool of Traditional Greek Dairy Products Regarding Specific Strain-Host Interactions. Probiotics & Antimicro. Prot. 10, 313–322 (2018). https://doi.org/10.1007/s12602-017-9311-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-017-9311-9