Abstract
Development of new materials with high hydrogen storage capacity and reversible hydrogen sorption performances under mild conditions has very high value in both fundamental and application aspects. In the past years, some new systems with metastable structures, such as ultra-fine nanocrystalline alloys, amorphous alloys, nanoglass alloys, immiscible alloys, high-entropy alloys, have been abundantly studied as hydrogen storage materials. Many new hydrogen storage properties either from the kinetics or thermodynamics aspects have been reported. In this review, recent advances of studies on metastable alloys for hydrogen storage applications have been comprehensively reviewed. The materials preparation methods to synthesize metastable hydrogen storage alloys are firstly reviewed. Afterwards, hydrogen storage properties of the metastable alloys are summarized and discussed, focusing on the unique kinetics and thermodynamics properties by forming of such unique metastable structures. For examples, superior hydrogenation kinetics and higher hydrogen storage capacity have been achieved in Mg-based amorphous and nanoglass alloys. Destabilized thermodynamics properties can be obtained in the immiscible Mg–Mn and Mg–Zr alloys. In addition to highlighting the recent achievements of metastable alloys in the field of hydrogen storage, the remaining challenges and trends of the emerging research are also discussed.
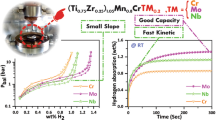
Graphical abstract

摘要
开发具有高储氢容量和可逆吸放氢性能的新型储氢材料具有重要的学术和应用价值。在过去几年中, 一些新型亚稳储氢材料 (如超细纳米晶合金、非晶合金、纳米玻璃合金、互不溶合金、高熵合金等) 受到学界关注, 这些亚稳储氢材料具有新的储氢特性。本文详细综述了亚稳储氢合金在储氢领域的应用研究进展。首先, 介绍了亚稳储氢合金的制备方法。其次, 重点阐述了各类亚稳储氢合金的独特亚稳结构以及由此形成的独特的储氢热力学和动力学性能。例如, 镁基非晶或者纳米玻璃合金具有优异的吸氢动力学性能和更高的储氢容量; 又如, Mg–Mn、Mg–Zr等互不溶合金的热力学稳定性会得到降低。最后, 在总结亚稳储氢合金最近研究成果基础上, 指出了亚稳储氢合金的挑战以及未来的发展方向。


Reproduced with permission from Ref. [16]. Copyright 2016, Elsevier

Reproduced with permission from Ref. [17]. Copyright 2018, Elsevier

Reproduced with permission from Ref. [117]. Copyright 2016, Elsevier

Reproduced with permission from Ref. [137]. Copyrights 2017, The Royal Society of Chemistry

Reproduced with permission from Ref. [146]. Copyright 2011, Elsevier

Similar content being viewed by others
References
Qin PL, Zeng K, Lan ZQ, Huang XT, Liu HZ, Guo J. Enhanced dydrogen storage properties of mg-al alloy catalyzed with reduced graphene oxide supported with LaClO. Chin J Rare Metals. 2020;44(5):499.
Hua W, Sun HH, Xu F, Wang JG. A review and perspective on molybdenum-based electrocatalysts for hydrogen evolution reaction. Rare Met. 2020;39(4):335.
Moradi R, Groth KM. Hydrogen storage and delivery: review of the state of the art technologies and risk and reliability analysis. Int J Hydrogen Energy. 2019;44(23):12254.
Hirscher M, Yartys VA, Baricco M, Bellosta von Colbe J, Blanchard D, Bowman RC, Broom DP, Buckley CE, Chang F, Chen P, Cho YW, Crivello JC, Cuevas F, David WIF, DenysRV, Dornheim M, Felderhoff M, Filinchuk Y, Froudakis GE, Grant DM, Gray EM, Hauback BC, He T, Humphries TD, Jensen TR, Kim S, Kojima Y, Latroche M, Li HW, Lototskyy MV, Makepeace JW, Møller KT, Naheed L, Ngene P, Noréus D, Nygård MM, Orimo Si, Paskevicius M, Pasquini L. Materials for hydrogen-based energy storage–past, recent progress and future outlook. J Alloys Compd. 2020;827:153548.
Lin HJ, Li HW, Shao H, Lu Y, Asano K. In situ measurement technologies on solid-state hydrogen storage materials: a review. Mater Today Energy. 2020;17:100468.
Ouyang L, Liu F, Wang H, Liu J, Yang XS, Sun L, Zhu M. Magnesium-based hydrogen storage compounds: a review. J Alloys Compd. 2020;832:154865.
Zhang YH, Huang G, Yuan ZM, Guo SH, Qi Y, Zhao DL. Electrochemical hydrogen storage behaviors of as-cast and spun RE–Mg–Ni–Co–Al-based AB2-type alloys applied to Ni–MH battery. Rare Met. 2020;39(2):181.
Jiang W, Wang H, Zhu M. AlH3 as a hydrogen storage material: recent advances, prospects and challenges. Rare Met. 2021;140(12):3337.
Vucht JHN, Kuijpers FA, Bruning HCAM. Reversible room-temperature absorption of large quantities of hydrogen by intermetallic compounds. Philips Research Report. 1970:133.
Buschow KH, Van Mal HH. Phase relations and hydrogen absorption in the lanthanum-nickel system. J Less Common Metals. 1972;29(2):203.
Reilly J, Wiswall R Jr. Formation and properties of iron titanium hydride. Inorg Chem. 1974;13(1):218.
Akiba E, Iba H. Hydrogen absorption by Laves phase related BCC solid solution. Intermetallics. 1998;6(6):461.
Reilly JJ, Wiswall RH. Reaction of hydrogen with alloys of magnesium and nickel and the formation of Mg2NiH4. Inorg Chem. 1968;7:2254.
Stampfer JF, Holley CE, Suttle JF. The magnesium-hydrogen system. J Am Chem Soc. 1960;82(14):3504.
Li J, Li B, Yu X, Zhao H, Shao H. Geometrical effect in Mg-based metastable nano alloys with BCC structure for hydrogen storage. Int J Hydrogen Energy. 2019;44(55):29291.
Lin HJ, He M, Pan SP, Gu L, Li HW, Wang H, Ouyang LZ, Liu JW, Ge TP, Wang DP, Wang WH, Akiba E, Zhu M. Towards easily tunable hydrogen storage via a hydrogen-induced glass-to-glass transition in Mg-based metallic glasses. Acta Mater. 2016;120:68.
Xu C, Lin HJ, Edalati K, Li W, Li L, Zhu Y. Superior hydrogenation properties in a Mg65Ce10Ni20Cu5 nanoglass processed by melt-spinning followed by high-pressure torsion. Scripta Mater. 2018;152:137.
Viano A, Stroud R, Gibbons P, McDowell A, Conradi M, Kelton K. Hydrogenation of titanium-based quasicrystals. Phys Rev B. 1995;51:12026.
Kunce I, Polanski M, Bystrzycki J. Microstructure and hydrogen storage properties of a TiZrNbMoV high entropy alloy synthesized using Laser Engineered Net Shaping (LENS). Int J Hydrogen Energy. 2014;39(18):9904.
Tan X, Wang L, Holt CMB, Zahiri B, Eikerling MH, Mitlin D. Phys. Chem. Chem. Phys. Body centered cubic magnesium niobium hydride with facile room temperature absorption and four weight percent reversible capacity. Phys Chem Chem Phys. 2012;14:10904.
Liu H, Zhang L, Ma H, Lu C, Luo H, Wang X, Huang X, Lan Z, Guo J. Aluminum hydride for solid-state hydrogen storage: structure, synthesis, thermodynamics, kinetics, and regeneration. J Energy Chem. 2021;52:428.
El-Eskandarany MS. Recent developments in the fabrication, characterization and implementation of MgH2-based solid-hydrogen materials in the Kuwait Institute for Scientific Research. RSC Adv. 2019;9:9907.
Zhang J, Yan S, Qu H. Recent progress in magnesium hydride modified through catalysis and nanoconfinement. Int J Hydrogen Energy. 2017;43(3):1545.
Zhang XL, Liu YF, Zhang X, Hu JJ. Empowering hydrogen storage performance of MgH2 by nanoengineering and nanocatalysis. Mater. Today Nano. 2020;9:100064.
Sakintuna B, Lamari-Darkrim F, Hirscher M. Metal hydride materials for solid hydrogen storage: a review. Int J Hydrogen Energy. 2007;32:1121.
Wang H, Lin HJ, Cai WT, Ouyang LZ, Zhu M. Tuning kinetics and thermodynamics of hydrogen storage in light metal element based systems—a review of recent progress. J Alloys Compd. 2016;658:280.
He T, Pachfule P, Wu H, Xu Q, Chen P. Hydrogen carriers. Nat Rev Mater. 2016;1:16059.
Zhao Q, Li Y, Song Y, Cui X, Sun D, Fang F. Fast hydrogen-induced optical and electrical transitions of Mg and Mg-Ni films with amorphous structure. Appl Phys Lett. 2013;102:161901.
Turnbull D. Metastable structures in metallurgy. Metall Mater Trans B. 1981;12:217.
Huot J, Ravnsbæk DB, Zhang J, Cuevas F, Latroche M, Jensen TR. Mechanochemical synthesis of hydrogen storage materials. Prog Mater Sci. 2013;58:30.
Orimo S, Ikeda K, Fujii H, Fujikawa Y, Kitano Y, Yamamoto K. Structural and hydriding properties of the Mg–Ni–H system with nano- and/or amorphous structures. Acta Mater. 1997;45:2271.
Zhong HC, Wang H, Liu JW, Sun DL, Zhu M. Altered desorption enthalpy of MgH2 by the reversible formation of Mg(In) solid solution. Scripta Mater. 2011;65:285.
Zhong HC, Lin HJ, Lu XJ, Cao CJ, Chen C, Sun JJ. New mechanism and improved kinetics of hydrogen absorption and desorption of Mg(In) solid solution alloy milling with CeF3. Int J Hydrogen Energy. 2019;44(43):23996.
Skripnyuk VM, Rabkin E. Mg3Cd: a model alloy for studying the destabilization of magnesium hydride. Int J Hydrogen Energy. 2012;37(14):10724.
Si TZ, Zhang JB, Liu DM, Zhang QA. A new reversible Mg3Ag–H2 system for hydrogen storage. J Alloys Compd. 2013;581:246.
Zaluski L, Zaluska A, Strom-Olsen JO. Hydrogen absorption in nanocrystalline Mg2Ni formed by mechanical alloying. J Alloys Compd. 1995;217(2):245.
Zaluski L, Zaluska A, Strom-Olsen JO. Nanocrystalline metal hydrides. J Alloys Compd. 1997;253–254:70.
Zaluska A, Zaluski L, Strom-Olsen JO. Nanocrystalline magnesium for hydrogen storage. J Alloys Compd. 1999;288:217.
Oelerich W, Klassen T, Bormann R. Metal oxides as catalysts for improved hydrogen sorption in nanocrystalline Mg-based materials. J Alloys Compd. 2001;315(1–2):237.
Barkhordarian G, Klassen T, Bormann R. Effect of Nb2O5 content on hydrogen reaction kinetics of Mg. J Alloys Compd. 2004;364(1–2):242.
Cui J, Wang H, Liu J, Ouyang L, Zhang Q, Sun D, Yao X, Zhu M. Remarkable enhancement in dehydrogenation of MgH2 by a nano-coating of multi-valence Ti-based catalysts. J Mater Chem A. 2013;18:5603.
Lu ZY, Yu HJ, Lu X, Song MC, Wu FY, Zheng JG, Yuan ZF, Zhang LT. Two-dimensional vanadium nanosheets as a remarkably effective catalyst for hydrogen storage in MgH2. Rare Met. 2021;40(11):3195.
Si TZ, Zhang XY, Feng JJ, Ding XL, Li YT. Enhancing hydrogen sorption in MgH2 by controlling particle size and contact of Ni catalysts. Rare Met. 2021;40(4):995.
Chen HS, Miller CE. A rapid quenching technique for the preparation of thin uniform films of amorphous solids. Rev Sci Instrum. 1970;41:1237.
Lin HJ, Zhang C, Wang H, Ouyang LZ, Zhu YF, Li QL, Wang WH, Zhu M. Controlling nanocrystallization and hydrogen storage property of Mg-based amorphous alloy via a gas-solid reaction. J Alloys Compd. 2016;685:272.
Lin HJ, Tang JJ, Yu Q, Wang H, Ouyang LZ, Zhao YJ, Liu JW, Wang WH, Zhu M. Symbiotic CeH2.73/CeO2 catalyst: a novel hydrogen pump. Nano Energy. 2014;9:80.
Zhang QA, Zhang LX, Wang QQ. Crystallization behavior and hydrogen storage kinetics of amorphous Mg11Y2Ni2 alloy. J Alloys Compd. 2013;551:376.
Révész Á, Gajdics M. Improved H-storage performance of novel Mg-based nanocomposites prepared by high-energy ball milling: a review. Energies. 2021;14(19):6400.
Lass EA. Hydrogen storage in rapidly solidified and crystallized Mg–Ni–(Y, La)–Pd alloys. Int J Hydrogen Energy. 2012;37(12):9716.
Denys RV, Poletaev AA, Maehlen JP, Solberg JK, Tarasov BP, Yartys VA. Nanostructured rapidly solidified LaMg11Ni alloy. II. In situ synchrotron X-ray diffraction studies of hydrogen absorption–desorption behaviours. Int J Hydrogen Energy. 2012;37(7):5710.
Zhang QA, Liu DD, Wang QQ, F Fang F, Sun DL, Ouyang LZ, Zhu M. Superior hydrogen storage kinetics of Mg12YNi alloy with a long-period stacking ordered phase. Scripta Mater. 2011;65(3):233.
Lass EA. Hydrogen storage measurements in novel Mg-based nanostructured alloys produced via rapid solidification and devitrification. Int J Hydrogen Energy. 2011;36(17):10787.
Denys RV, Poletaev AA, Solberg JK, Tarasov BP, Yartys VA. LaMg11 with a giant unit cell synthesized by hydrogen metallurgy: crystal structure and hydrogenation behavior. Acta Mater. 2010;58(7):2510.
Spassov T, Lyubenova L, Köster U, Baró MD. Mg–Ni–RE nanocrystalline alloys for hydrogen storage. Mat Sci Eng A. 2004;375–377:794.
Spassov T, Solsona P, Suriñach S, Baró MD. Nanocrystallization in Mg83Ni17−xYx (x = 0, 7.5) amorphous alloys. J Alloys Compd. 2002;345(1–2):123.
Spassov T, Köster U. Thermal stability and hydriding properties of nanocrystalline melt-spun Mg63Ni30Y7 alloy. J Alloys Compd. 1998;279(2):279.
Stroud RM, Viano AM, Gibbons PC, Kelton KF, Misture ST. Stable Ti-based quasicrystal offers prospect for improved hydrogen storage. Appl Phys Lett. 1996;69(20):2998.
Hu W, Wang J, Wang L, Wu Y, Wang L. Electrochemical hydrogen storage in (Ti1−xVx)2Ni (x=0.05–0.3) alloys comprising icosahedral quasicrystalline phase. Electrochim Acta. 2009;54:2770.
Hu W, Niu XD, Watada M, Kawabe Y, Wu YM, Wang LD, Wang LM. Electrochemical hydrogen storage in Ti1.6V0.4Ni1−xCox icosahedral quasicrystalline alloys. ChemPhysChem. 2010;11(1):295.
Badding JV, Parker LJ, Nesting DC. High pressure synthesis of metastable materials. J Solid State Chem. 1995;117(2):229.
Vajeeston P, Ravindran P, Kjekshus A, Fjellvåg H. Pressure-induced structural transitions in MgH2. Phys Rev Lett. 2002;89(17–21):175506.
Kyoi D, Sato T, Rönnebro E, Kitamura N, Ueda A, Ito M, Katsuyama S, Hara S, Noréus D, Sakai T. A new ternary magnesium–titanium hydride Mg7TiHx with hydrogen desorption properties better than both binary magnesium and titanium hydrides. J Alloys Compd. 2004;372(1–2):213.
Kyoi D, Sato T, Rönnebro E, Tsuji Y, Kitamura N, Ueda A, Ito M, Katsuyama S, Hara S, Noréus D, Sakai T. A novel magnesium–vanadium hydride synthesized by a gigapascal-high-pressure technique. J Alloys Compd. 2004;375(1–2):253.
Kyoi D, Kitamura N, Tanaka H, Ueda A, Tanase S, Sakai T. Hydrogen desorption properties of FCC super-lattice hydride Mg7NbHx prepared by ultra-high pressure techniques. J Alloys Compd. 2007;428(1–2):268.
Kyoi D, Sakai T, Kitamura N, Ueda A, Tanase S. Synthesis of FCC Mg–Zr and Mg–Hf hydrides using GPa hydrogen pressure method and their hydrogen-desorption properties. J Alloys Compd. 2008;463(1–2):311.
Kyoi D, Sakai T, Kitamura N, Ueda A, Tanase S. Synthesis of FCC Mg–Ta hydrides using GPa hydrogen pressure method and their hydrogen-desorption properties. J Alloys Compd. 2008;463(1–2):306.
Kamegawa A, Goto Y, Kakuta H, Takamura H, Okada M. High-pressure synthesis of novel hydrides in Mg–RE–H systems (RE = Y, La, Ce, Pr, Sm, Gd, Tb, Dy). J Alloy Compd. 2006;408–412:284.
Selvam P, Yvon K. Synthesis of Mg2FeH6, Mg2CoH5 and Mg2NiH4 by high-pressure sintering of the elements. Int J Hydrogen Energy. 1991;16(9):615.
Valiev R, Valiev R. Nanostructuring of metals by severe plastic deformation for advanced properties. Nat Mater. 2004;3:511.
Valiev RZ, Islamgaliev RK, Alexandrov IV. Bulk nanostructured materials from severe plastic deformation. Prog Mater Sci. 2000;45(2):103.
Skripnyuk VM, Rabkin E, Estrin Y. Lapovok R, Acta Mater. The effect of ball milling and equal channel angular pressing on the hydrogen absorption/desorption properties of Mg–4.95 wt% Zn–0.71 wt% Zr (ZK60) alloy. Acta Mater. 2004:52(2)405.
Edalati K, Horita Z. A review on high-pressure torsion (HPT) from 1935 to 1988. Mater Sci Eng A. 2016;652:325.
Edalati K, Emami H, Staykov A, Smith DJ, Akiba E, Horita Z. Formation of metastable phases in magnesium–titanium system by high-pressure torsion and their hydrogen storage performance. Acta Mater. 2015;99:150.
Révész Á, Kis-Tóth Á, Varga LK, Schafler E, Bakonyi I, Spassov T. Hydrogen storage of melt-spun amorphous Mg65Ni20Cu5Y10 alloy deformed by high-pressure torsion. Int J Hydrogen Energy. 2012;37(7):5769.
Cantor B, Cahn RW. Metastable alloy phases by co-sputtering. Acta Metall Sin. 1976;24:845.
Mooij LPA, Baldi A, Boelsma C, Shen K, Wagemaker M, Pivak Y, Schreuders H, Griessen R, Dam B. Interface energy controlled thermodynamics of nanoscale metal hydrides. Adv Energy Mater. 2011;1:754.
Chung CJ, Lee SC, Groves JR, Brower EN, Sinclair R, Clemens BM. Interfacial alloy hydride destabilization in Mg/Pd thin films. Phys Rev Lett, 2012;108:106102.
Bannenberg LJ, Boelsma C, Asano K, Schreuders H, Dam B. Metal hydride based optical hydrogen sensors. J Phys Soc Japan. 2020;89:051003.
Baldi A, Gonzalez-Silveira M, Palmisano V, Dam B, Griessen R. Destabilization of the Mg–H system through elastic constraints. Phys Rev Lett. 2009;102:226102.
Qu JL, Sun B, Zheng J, Yang R, Wang YT, Li XG. Hydrogen desorption properties of Mg thin films at room temperature. Power Sources. 2010;195:1190.
Zhang LT, Yan NH, Yao ZD, Sun Z, Lu X, Nyahuma FM, Zhu RH, Tu GP, Chen LX. Remarkably improved hydrogen storage properties of carbon layers covered nanocrystalline Mg with certain air stability. Int J Hydrogen Energy. 2020;45:28134.
Yan NH, Lu X, Lu ZY, Yu HJ, Wu FY , Zheng JG, Wang XZ, Zhang LT. Enhanced hydrogen storage properties of Mg by the synergistic effect of grain refinement and NiTiO3 nanoparticles. J Magnes Alloy. https://doi.org/10.1016/j.jma.2021.03.014.
Shao HY, Xu HR, Wang YT, Li XG. Preparation and hydrogen storage properties of Mg2Ni intermetallic nanoparticles. Nanotechnology. 2004;15:269.
Vermeulen P, Niessen RAH, Borsa DM, Dam B, Griessen R, Notten PHL. Effect of the deposition technique on the metallurgy and hydrogen storage characteristics of metastable MgyTi1−y thin films. Solid-State Lett. 2006;9(11): A520.
Shen CQ, Aguey-Zinsou KF. Can γ-MgH2 improve the hydrogen storage properties of magnesium? J Mater Chem A. 2017;18:644.
Xiao XZ, Liu Z, Saremi-Yarahmadi S, Gregory DH. Facile preparation of β-/γ-MgH2 nanocomposites under mild conditions and pathways to rapid dehydrogenation. Phys chem chem phys. 2016;18:10492.
Klement W, Willens RH, Duwez POL. Non-crystalline structure in solidified gold-silicon alloys. Nature. 1960;187:869.
Inoue A, Ohtera K, Kita K, Masumoto T. New amorphous Mg–Ce–Ni alloys with high strength and good ductility. Jap J Appl Phys. 1988;27:L2248.
Inoue A, Ohtera K, Kohinata M, Tsai AP, T. Masumoto. Glass transition behavior of Al- and Mg-based amorphous alloys. J Non-Crystalline Solids. 1990;117–118:712.
Spassov T, Köster U. Hydrogenation of amorphous and nanocrystalline Mg-based alloys. J Alloy Compd. 1999;287:243.
Tanaka K, Kanda Y, Furuhashi M, Saito K, Kuroda K, Saka H. Improvement of hydrogen storage properties of melt-spun Mg–Ni–RE alloys by nanocrystallization. J Alloy Compd. 1999;293–295:521.
Wu Y, Solberg JK, Yartys VA. The effect of solidification rate on microstructural evolution of a melt-spun Mg–20Ni–8Mm hydrogen storage alloy. J Alloy Compd. 2007;446–447:178.
Wu Y, Han W, Zhou SX, Lototsky MV, Solberg JK, Yartys VA. Microstructure and hydrogenation behavior of ball-milled and melt-spun Mg–10Ni–2Mm alloys. J Alloy Compd. 2008;466:176.
Teresiak A, Uhlemann M, Gebert A, Thomas J, Eckert J, Schultz L. Formation of nanostructured LaMg2Ni by rapid quenching and intensive milling and its hydrogen reactivity. J Alloy Compd. 2009;481:144.
Kalinichenka S, Rontzsch L, Baehtz C, Kieback B. Hydrogen desorption kinetics of melt-spun and hydrogenated Mg90Ni10 and Mg80Ni10Y10 using in situ synchrotron, X-ray diffraction and thermogravimetry. J Alloy Compd. 2010;496:608.
Kalinichenka S, Rontzsch L, Baehtz C, Weißgärber T, Kieback B. Hydrogen desorption properties of melt-spun and hydrogenated Mg-based alloys using in situ synchrotron X-ray diffraction and TGA. J Alloy Compd. 2011;509:S629.
Kalinichenka S, Rontzsch L, Kieback B. Structural and hydrogen storage properties of melt-spun Mg–Ni–Y alloys. Int J Hydrogen Energy. 2009;34:7749.
Zhang YH, Li BW, Ren HP, Guo SH, Wu ZW, Wang XL. An investigation on the hydrogen storage characteristics of the melt-spun nanocrystalline and amorphous Mg20−xLaxNi10 (x = 0, 2) hydrogen storage alloys. Mater Chem Phys. 2009;115:328.
Lin HJ, Ouyang LZ, Wang H, Zhao DQ, Wang WH, Sun DL, Zhu M. Hydrogen storage properties of Mg–Ce–Ni nanocomposite induced from amorphous precursor with the highest Mg content. Int J Hydrogen Energy. 2012;37:14329.
Kalinichenka S, Röntzsch L, Riedl T, Gemming T, Weißgärber T, Kieback B. Microstructure and hydrogen storage properties of melt-spun Mg–Cu–Ni–Y alloys. Int J Hydrogen Energy. 2011;36:1592.
Zhang QA, Jiang CJ, Liu DD. Comparative investigations on the hydrogenation characteristics and hydrogen storage kinetics of melt-spun Mg10NiR (R = La, Nd and Sm) alloys. Int J Hydrogen Energy. 2012;37:10709.
Wu Y, Lototsky MV, Solberg JK, Yartys VA, Han W, Zhou SX. Microstructure and novel hydrogen storage properties of melt-spun Mg–Ni–Mm alloys. J Alloys Compd. 2009;477:262.
Lin HJ, He LQ, Zhang P, Zhang ZG, Pan SP, Li W. Tailoring hydrogen storage properties of amorphous Mg65Cu25Y10 alloy via minor alloying addition of Ag. Intermetallics. 2018;97:22.
Lin HJ, Xu C, Gao M, Ma ZL, Meng YY, Li LQ, Hu XH, Zhu YF, Pan SP, Li W. Hydrogenation properties of five-component Mg60Ce10Ni20Cu5X5 (X = Co, Zn) metallic glasses. Intermetallics. 2019;108:94.
Ismail N, Uhlemann M, Gebert A, Eckert J. Hydrogenation and its effect on the crystallisation behaviour of Zr55Cu30Al10Ni5 metallic glass. J Alloys Compd. 2000;298:146.
Révész Á, Kis-Tóth Á, Varga LK, Lábár JL, Spassov T. High glass forming ability correlated with microstructure and hydrogen storage properties of a Mg–Cu–Ag–Y glass. Int J Hydrogen Energy. 2014;39:9230.
Jing J, Krämer A, Birringer R, Gleiter H, Gonser U. Modified atomic structure in a Pd70Fe3Si27 nanoglass: a Mössbauer study. J Non-Crystalline Solids. 1989;113:167.
Wang CM, Wang D, Mu XK, Goel S, Feng T, Ivanisenko Y, Hahn H, Gleiter H. Surface segregation of primary glassy nanoparticles of Fe90Sc10 nanoglass. Mater Lett. 2016;181:248.
Fang JX, Vainio U, Puff W, Würschum R, Wang XL, Wang D, Ghafari M, Jiang F, Sun J, Hahn H, Gleiter H. Atomic structure and structural stability of Sc75Fe25 Nanoglasses. Nano Lett. 2012;12:458.
Gleiter H. Nanoglasses: a new kind of noncrystalline materials. Beilstein J Nanotech. 2013;4:517.
Wang JQ, Chen N, Liu P, Wang Z, Louzguine-Luzgin DV, Chen MW, Perepezko JH. The ultrastable kinetic behavior of an Au-based nanoglass. Acta Mater. 2014;79:30.
Chen N, Wang D, Feng T, Kruk R, Yao KF, Louzguine-Luzgin DV, Hahn H, Gleiter H. A nanoglass alloying immiscible Fe and Cu at the nanoscale. Nanoscale. 2015;7:6607.
Xu C, Lin H, Edalati K, Li W, Li L, Zhu Y. Superior hydrogenation properties in a Mg65Ce10Ni20Cu5 nanoglass processed by melt-spinning followed by high-pressure torsion. Scr Mater. 2018;152:137.
Lee SH, Choi SB, E. Eisuke I, Kim JY. Structural stability and kinetics of hydrogen in TiZrNi quasicrystals. J Nanosci Nanotechno. 2010;10:7680.
Lee SH, Jo YS, Kim JY. Hydrogen absorption and structural analysis of TiZrNiV quasicrystals. J Nanosci Nanotechno. 2014;14:9373.
Jo YS, Lee SH, Shin HS, Kim JY. Analysis of structure and P–C–T curve of hydrogenated Ti53Zr27−xNi20Pdx quasicrystals. J Nanosci Nanotechno. 2013;13:7959.
Balcerzak M. Hydrogenation study of nanostructured Ti–Zr–Ni alloys. J Energy Storage. 2016;8:6.
Huang H, Meng DQ, Lai XC, Liu TW, Long Y, Hu QM. Structure of bergman-type W–TiZrNi approximants to quasicrystal, analyzed by lattice inversion method. J Phys: Condens. Mat. 2014;26:315003.
Luo XL, Grant DM, Walker GS. Hydrogen storage properties for Mg–Zn–Y quasicrystal and ternary alloys. J Alloys Compd. 2015;645:S23.
Yeh JW, Chen SK, Lin SJ, Gan JY, Chin TS, Shun TT, Tsau CH, Chang SY. Nanostructured high-entropy alloys with multiple principal elements: novel alloy design concepts and outcomes. Adv Eng Mater. 2004;6(5):299.
Qin YC, Wang FQ, Wang XM, Wang MW, Zhang WL, An WK, Wang XP, Ren YL, Zheng X, Lv DC. Noble metal-based high-entropy alloys as advanced electrocatalysts for energy conversion. Rare Met. 2021;40(9):2354.
Sahlberg M, Karlsson D, Zlotea C, Jansson U. Superior hydrogen storage in high entropy alloys. Sci Rep. 2016;6:36770.
Zlotea C, Sow MA, Ek G, Couzinié JP, Perrière L, Guillot I, Bourgon J, Møller KT, Jensen TR, Akiba E, Sahlberg M. Hydrogen sorption in TiZrNbHfTa high entropy alloy. J Alloys Compd. 2019;775:667.
de Marco MO, Li Y, Li HW, Edalati K, Floriano R. Mechanical synthesis and hydrogen storage characterization of MgVCr and MgVTiCrFe high-entropy alloy. Adv Eng Mater. 2020;22(2):1901079.
Edalati P, Floriano R, Mohammadi A, Li Y, Zepon G, Li HW, Edalati K. Reversible room temperature hydrogen storage in high-entropy alloy TiZrCrMnFeNi. Scripta Mater. 2020;178: 387.
Zhang C, Song A, Yuan Y, Wu Y, Zhang P, Lu Z, Song X. Study on the hydrogen storage properties of a TiZrNbTa high entropy alloy. Int J Hydrogen Energy. 2020;45(8):5367.
Cardoso KR, Roche V, Jorge Jr AM, Antiqueira FJ, Zepon G, Champion Y. Hydrogen storage in MgAlTiFeNi high entropy alloy. J Alloys Compd. 2021;858 :158357.
Karlsson D, Ek G, Cedervall J, Zlotea C, MØller KT, Hansen TC, Bednarčík, Paskevicius M, SØrby MH, Jensen TR, Jansson U, Sahlberg M. Structure and hydrogenation properties of a HfNbTiVZr high-entropy alloy. Inorg. Chem. 2018;57(4): 2103.
Dong H, Huang C, Moser D, Noréus D, Zhu M. Structure and stability of high pressure synthesized MgTM2H6 (TM = Zr, Nb) hydrides. Acta Mater. 2015;96:237.
Shao H, Asano K, Enoki H, Akiba E. Fabrication hydrogen storage properties and mechanistic study of nanostructured Mg50Co50 body-centered cubic alloy. Scripta Mater. 2009;60(9):818.
Zhang Y, Tsushio Y, Enoki H, Akiba E. The study on binary Mg–Co hydrogen storage alloys with BCC phase. J Alloys Compd. 2005;393(1–2):147.
Edalati K, Emami H, Ikeda Y, Iwaoka H, Tanaka I, Akiba E, Horita Z. New nanostructured phases with reversible hydrogen storage capability in immiscible magnesium–zirconium system produced by high-pressure torsion. Acta Mater. 2016;108:293.
Gómez EIL, Edalati K, Coimbrão DD, Antiqueira FJ, Zepon G, Cubero-Sesin JM, Botta WJ. FCC phase formation in immiscible Mg–Hf (magnesium–hafnium) system by high-pressure torsion. AIP Advances. 2020;10:055222.
Anastasopol A, Pfeiffer TV, Middelkoop J, Lafont U, Canales-Perez RJ, Schmidt-Ott A, Mulder FM, Eijt SWH. Reduced enthalpy of metal hydride formation for Mg–Ti nanocomposites produced by spark discharge generation. J Am Chem Soc. 2013;135(21):7891.
Calizzi M, Venturi F, Ponthieu M, Cuevas F, Morandi V, Perkisas T, Bals S, Pasquini L. Gas-phase synthesis of Mg–Ti nanoparticles for solid-state hydrogen storage. Phys Chem Chem Phys. 2016;18:141.
Lu Y, Kim H, Sakaki K, Hayashi S, Jimura K, Asano K. Destabilizing the dehydrogenation thermodynamics of magnesium hydride by utilizing the immiscibility of Mn with Mg. Inorg Chem. 2019;58(21):14600.
Lu Y. Kim H, Jimura K, Hayashi S, Sakaki K, Asano K.Strategy of thermodynamic and kinetic improvements for Mg hydride nanostructured by immiscible transition metals. J Power Sources. 2021;494:229742.
Ding X, Li Y, Fang F, Sun D, Zhang Q. Hydrogen-induced magnesium–zirconium interfacial coupling: enabling fast hydrogen sorption at lower temperatures. J Mater Chem A. 2017;5:5067.
Asano K, Westerwaal RJ, Anastasopol A, Mooij LPA, Boelsma C, Ngene P, Schreuders H, Eijt SWH, Dam B. Destabilization of Mg hydride by self-organized nanoclusters in the immiscible Mg–Ti system. J Phys Chem C. 2015;119(22):12157.
Asano K, Westerwaal RJ, Schreuders H, Dam B. Enhancement of destabilization and reactivity of Mg hydride embedded in immiscible Ti matrix by addition of Cr: Pd-free destabilized Mg hydride. J Phys Chem C. 2017;121(23):12631.
Vermeulen P, Niessen RAH, Notten PHL. Hydrogen storage in metastable MgyTi(1−y) thin films. Electrochem Commun. 2006;8(1):27.
Lu Y, Asano K, Schreuders H, Kim H, Sakaki K, Machida A, Watanuki T, Dam B. Nanostructural perspective for destabilization of Mg hydride using the immiscible transition metal Mn. Inorg Chem. 2021;60(19):15024.
Zheng S, Wang K, Oleshko VP, Bendersky LA. Mg–Fe thin films: a phase-separated structure with fast kinetics of hydrogenation. J Phys Chem C. 2012;116(40):21277.
Ngene P, Longo A, Mooij L, Bras W, Dam B. Metal-hydrogen systems with an exceptionally large and tunable thermodynamic destabilization. Nat Commun. 2017;8:1846.
Graetz J. Metastable metal hydrides for hydrogen storage. ISRN Mater Sci. 2012; 2012:863025.
Yu M, Zhu Z, Li HP, Yan QL. Advanced preparation and processing techniques for high energy fuel AlH3. Chem Eng J. 2021;421(1):129753.
Graetz J, Reilly JJ, Yartys VA, Maehlen JP, Bulychev BM, Antonov VE, Tarasov BP, Gabis IE. Aluminum hydride as a hydrogen and energy storage material: past, present and future. J Alloys Compd. 2011;509(2):S517.
Finholt AE, Bond AC, Schlesinger HI. Lithium aluminum hydride, aluminum hydride and lithium gallium hydride, and some of their applications in organic and inorganic chemistry. J Am Chem Soc. 1947;69(5):1199.
Sandrock G, Reilly J, Graetz J, Zhou WM, Johnson J, Wegrzyn J. Accelerated thermal decomposition of AlH3 for hydrogen-fueled vehicles. Appl Phys A. 2005;80:687.
Brower FM, Matzek NE, Reigler PF, Rinn HW, Roberts CB, Schmidt DL, Snover JA, Terada K. Preparation and properties of aluminum hydride. J Am Chem Soc. 1976;98:2450.
Bulychev BM, Verbetskii VN, Sizov AI, Zvukova TM, Genchel VK, Fokin VN. Non-solvated aluminum hydride. Crystallization from diethyl ether-benzene solutions. Russ Chem Bull. 2007: 56:1305.
Turley JW, Rinn HW. Crystal structure of aluminum hydride. Inorg Chem. 1969;8(1):18.
Brinks HW, Istad-Lem A, Hauback BC. Mechanochemical synthesis and crystal structure of α’-AlD3 and α-AlD3. J Phys Chem B. 2006;110:25833.
Brinks HW, Langley W, Jensen CM, Graetz J, Reilly JJ, Hauback BC. Synthesis and crystal structure of β-AlD3. J Alloys Compd. 2007;433(1–2):180.
Yartys VA, Denys RV, Maehlen JP, Frommen C, Fichtner M, Bulychev BM, Emerich H. Double-bridge bonding of aluminium and hydrogen in the crystal structure of γ-AlH3. Inorg Chem. 2007;46:1051.
Wang L, Rawal A, Quadir MZ, Aguey-Zinsou KF. Formation of aluminium hydride (AlH3) via the decomposition of organoaluminium and hydrogen storage properties. Int J Hydrogen Energy. 2018;43(34):16749.
Liu H, Wang X, Dong Z, Cao G, Liu Y, Chen L, Yan M. Dehydriding properties of γ-AlH3. Int J Hydrogen Energy. 2013;38(25):10851.
Sandrock G, Reilly J, Graetz J. Zhou WM, Johnson J, Wegrzyn J, Alkali metal hydride doping of α-AlH3 for enhanced H2 desorption kinetics - ScienceDirec. J Alloys Compd. 2006;421(1–2):185
Nakagawa Y, Lee CH, Matsui K, Kousaka K, Isobe S, Hashimoto N. Doping effect of Nb species on hydrogen desorption properties of AlH3. J Alloys Compd. 2018;734:55.
Luo Y, Wang Q, Li J, Xu F, Sun L, Zou Y, Chu H, Li B, Zhang K. Enhanced hydrogen storage/sensing of metal hydrides by nano-modification. Mater Today Nano. 2020;9:100071.
Wang L, Rawal A, Aguey-Zinsou KF. Hydrogen storage properties of nanoconfined aluminium hydride (AlH3). Chem Eng Sci. 2019;194:64.
Chen T, Liu H, Xu L, Li S, Ge H, Wang X. Effects of Nb-based additives on hydrogen desorption properties of AlH3. J Mater Sci Eng. 2017;35:9.
Paskevicius M, Sheppard DA, Buckley CE. Characterisation of mechanochemically synthesised alane (AlH3) nanoparticles. J Alloys Compd. 2009;487(1–2):370.
Hlova IZ, Gupta S, Goldston JF, Kobayashi T, Pruski M, Pecharsky VK. Dry mechanochemical synthesis of alane from LiH and AlCl3. Faraday Discuss. 2014;170:137.
Zidan R, Garcia-Diaz BL, Fewox CS, Stowe AC, Gray JR, Harter AG. Aluminium hydride: a reversible material for hydrogen storage. Chem Commun. 2009;40(25):3717.
Crouch-Baker S. Use of fluidized-bed electrode reactors for alane production. U.S. Patent. 2016–1–5: 9,228,267.
Graetz J, Chaudhuri S, Wegrzyn J, Celebi Y, Johnson JR, Zhou W, Reilly JJ. Direct and reversible synthesis of AlH3−triethylenediamine from Al and H2. J Phys Chem C. 2007;111(51):19148.
Lacina D, Wegrzyn J, Reilly J. Regeneration of aluminium hydride using dimethylethylamine. Energy Environ Sci. 2010;3(8):1099.
Ahluwalia RK, Hua TQ, Peng JK. Automotive storage of hydrogen in alane. Int J Hydrogen Energy. 2009;34(18):7731.
Grew KN, Brownlee ZB, Shukla KC. Assessment of alane as a hydrogen storage media for portable fuel cell power source. J Power Sources. 2012;217:417.
Thampan T, Atwater T, Cook C. Hydrogen generation from aluminum hydride for wearable polymer electrolyte membrane fuel cells. Int J Hydrogen Energy. 2016;41(22):9402.
Thampan T, Shah D, Cook C. Development and evaluation of portable and wearable fuel cells for soldier use. J Power Sources. 2014;259:276.
Bogdanovic B, Schwickardi M. Ti-doped alkali metal aluminium hydrides as potential novel reversible hydrogen storage materials. J Alloys Compd. 1997;253:1.
Fichtner M, Fuhr O. Synthesis and structures of magnesium alanate and two solvent adducts. J Alloys Compd. 2002;345(1–2):286.
Løvvik OM, Opalka SM, Brinks HW. Crystal structure and thermodynamic stability of the lithium alanates LiAlH4 and Li3AlH6. Phys Rev B, 2004;69:134117.
Mamatha A, Bogdanovic B, Felderhoff M, Pommerin A, Schmidt W, Schuth F, Weidenthaler C. Mechanochemical preparation and investigation of properties of magnesium, calcium and lithium–magnesium alanates. J Alloys Compd. 2006;407(1–2):78.
Orimo SI, Nakamori Y, Eliseo JR, Zuttel A, Jensen CM. Complex hydrides for hydrogen storage. Chem Rev. 2007;107(10):4111.
auback BC, Brinks HW, Fjellvg HJ. Accurate structure of LiAlD4 studied by combined powder neutron and X-ray diffraction. J Alloys Compd. 2002;346(1–2):184.
Fossdal A, Brinks HW, Fichtner M, Hauback BC. Determination of the crystal structure of Mg(AlH4)2 by combined X-ray and neutron diffraction. J Alloys Compd. 2005;387(1–2):47.
Løvvik OM, Crystal structure of Ca(AlH4)2 predicted from density-functional band-structure calculations. Phys Rev B. 2005;71:144111.
Komiya K, Morisaku N, Shinzato Y. Synthesis and dehydrogenation of M(AlH4)2 (M = Mg, Ca). J Alloys Compd. 2007;446–447:237.
Kabbour H, Ahn CC, Hwang SJ, Bowman RC, Graetz J. Direct synthesis and NMR characterization of calcium alanate. J Alloys Compd. 2007;446–447:264.
Andreasen A. Effect of Ti-doping on the dehydrogenation kinetic parameters of lithium aluminum hydride. J Alloys Compd. 2006;419(1–2):40.
Fichtner M, Fuhr O, Kircher O. Magnesium alanate–a material for reversible hydrogen storage. J Alloys Compd. 2003;356–357:418.
Fichtner M, Engel J, Fuhr O. The structure of magnesium alanate. Inorg Chem. 2003;42:7060.
Wei S, Xue S, Huang C. Multielement synergetic effect of NiFe2O4 and h-BN for improving the dehydrogenation properties of LiAlH4. Inorg Chem Front. 2021;8:3111.
Zhao L, Xu F, Zhang C, Wang Z, Ju H, Gao X, Zhang X, Sun L, Liu Z. Enhanced hydrogen storage of alanates: recent progress and future perspectives. Prog Nat Sci Mater Int. 2021;31(2):165.
Ismail M, Sazelee NA, Ali NA. Catalytic effect of SrTiO3 on the dehydrogenation properties of LiAlH4. J. Alloys. Compd. 2021;855:157475.
Hao CA, Jz A, Xxa C. Ultra-fast dehydrogenation behavior at low temperature of LiAlH4 modified by fluorographite. Int J Hydrogen Energy. 2020;45(52):28123.
Ali NA, Sazelee N, Yahya MS, Ismail M. Influence of K2NbF7 catalyst on the desorption behavior of LiAlH4. Front Chem. 2020;8:457.
Li ZL, Zhai FQ, Qiu HC, Wan Q, Li P, Qu XH. Dehydrogenation characteristics of ZrC-doped LiAlH4 with different mixing conditions. Rare Met. 2020;39(4):383.
Xia Y, Zhang H, Sun Y. Dehybridization effect in improved dehydrogenation of LiAlH4 by doping with two-dimensional Ti3C2. Mater Today Nano, 2019;8:100054.
Sulaiman NN, Ismail M, Timmiati SN. Improved hydrogen storage performances of LiAlH4 + Mg(BH4)2 composite with TiF3 addition. Int J Energy Res. 2021;45:2882.
Cheng L, Xu B, Li X. The roles of native defects and transition metal additives in the dehydrogenation mechanism of Mg(AlH4)2. Int J Hydrogen Energy. 2020;45(35):17625.
Wang J, Ebner AD, Ritter JA. Physiochemical pathway for cyclic dehydrogenation and rehydrogenation of LiAlH4. J Am Chem Soc. 2006;128(17):5949.
Graetz J, Wegrzyn J, Reilly JJ. Regeneration of lithium aluminum hydride. J Am Chem Soc. 1994;130(52):17790.
Liu X, Mcgrady GS, Langmi HW. Facile cycling of Ti-doped LiAlH4 for high performance hydrogen storage. J Am Chem Soc. 2009;131(14):5032.
Acknowledgements
This work was financially supported by Guangdong Basic and Applied Basic Research Foundation (No. 2019A1515011985), the National Natural Science Foundation of China (Nos. 52071157, 51801078, 52001070 and 52001079), the Natural Science Foundation of Jiangsu Province (No. BK20180986), the Natural Science Foundation of Guangxi Province (No. 2019GXNSFBA185004), Guangzhou Science and Technology Association Young Talent Lifting Project (No. X20200301071) and the Open Fund of the Guangdong Provincial Key Laboratory of Advance Energy Storage Materials (No. AESM202102).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Lin, HJ., Lu, YS., Zhang, LT. et al. Recent advances in metastable alloys for hydrogen storage: a review. Rare Met. 41, 1797–1817 (2022). https://doi.org/10.1007/s12598-021-01917-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-021-01917-8