Abstract
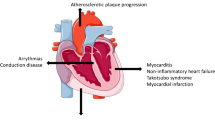
Immune-related adverse events occurring in the heart (cardiac immune-related adverse events; irAEs) by immune checkpoint inhibitors (ICIs) include myocarditis, arrhythmia, conduction disturbance, pericardial diseases, and takotsubo cardiomyopathy. Cardiac irAEs are rare but life-threatening. In cardio-oncology, the study of cardiac disorders caused by cancer treatment has recently attracted attention, and such studies may elucidate the pathophysiology of cardiac irAEs and contribute to management strategies. This review discusses the pathogenic mechanisms underlying cardiac irAEs and the role of echocardiography in patients treated with ICIs.


Similar content being viewed by others
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Onishi T, Fukuda Y, Miyazaki S, et al. Practical guidance for echocardiography for cancer therapeutics-related cardiac dysfunction. J Echocardiogr. 2021;19:1–20.
Okura Y, Ozaki K, Tanaka H, et al. The impending epidemic of cardiovascular diseases in patients with cancer in Japan. Circ J. 2019;83:2191–202.
Ball S, Ghosh RK, Wongsaengsak S, et al. Cardiovascular toxicities of immune checkpoint inhibitors. J Am Coll Cardiol. 2019;74:1714–27.
Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6:38.
Takahashi T, Tagami T, Yamazaki T, et al. Immunologic self-tolerance maintained by CD25(+) CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–10.
Kadowaki H, Akazawa H, Ishida J, Komuro I. Mechanisms and management of immune checkpoint inhibitor-related cardiac adverse events. JMA J. 2021;4:91–8.
Tajiri K, Aonuma K, Sekine I. Immune checkpoint inhibitor-related myocarditis. Jpn J Clin Oncol. 2018;48:7–12.
Salem JE, Manouchehri A, Moey M, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19:1579–89.
Ederhy S, Dolladille C, Thuny F, et al. Takotsubo syndrome in patients with cancer treated with immune checkpoint inhibitors: a new adverse cardiac complication. Eur J Heart Fail. 2019;21:945–7.
Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375:1749–55.
Mahmood SS, Fradley MG, Cohen HV, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71:1755–64.
Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4:1721–8.
Escudier M, Cautela J, Malissen N, et al. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation. 2017;136:2085–7.
Hu JR, Florido R, Lipson EJ, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc Res. 2019;115:854–68.
Palaskas N, Morgan J, Daigle T, et al. Targeted cancer therapies with pericardial effusions requiring pericardiocentesis focusing on immune checkpoint inhibitors. Am J Cardiol. 2019;123:1351–7.
Lyon AR, López-Fernández T, Couch LS, et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022;43:4229–361.
Rini B, Moslehi JJ, Bonaca M, et al. Prospective cardiovascular surveillance of immune checkpoint inhibitor-based combination therapy in patients with advanced renal cell cancer: data from the phase 3 JAVELIN Renal 101 trial. J Clin Oncol. 2022;40:1929–38.
Awadalla M, Mahmood SS, Groarke JD, et al. Global longitudinal strain and cardiac events in patients with immune checkpoint inhibitor-related myocarditis. J Am Coll Cardiol. 2020;75:467–78.
Tanabe J, Watanabe N, Endo A, et al. Asymptomatic immune checkpoint inhibitor-associated myocarditis. Intern Med. 2021;60:569–73.
Tamura Y, Tamura Y, Takemura R, et al. Longitudinal strain and troponin I elevation in patients undergoing immune checkpoint inhibitor therapy. JACC CardioOncol. 2022;4:673–85.
Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2018;36:1714–68.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Kazuaki Tanabe has received lecture fees from Otsuka Pharmaceutical Co, Ltd. Dr. Junya Tanabe declares that he has no conflict of interests.
Human rights statements and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent was obtained from the patient for being included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tanabe, K., Tanabe, J. Role of echocardiography in patients treated with immune checkpoints inhibitors. J Echocardiogr 21, 145–148 (2023). https://doi.org/10.1007/s12574-023-00621-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12574-023-00621-z