Abstract
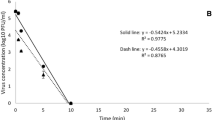
Surface disinfection, as part of environmental hygiene practices, is an efficient barrier to gastroenteritis transmission. However, surface disinfectants may be difficult to obtain in remote, resource-limited, or disaster relief settings. Electrochemical oxidants (ECO) are chlorine-based disinfectants that can be generated using battery power to electrolyze brine (NaCl) solutions. Electrolysis generates a mixed-oxidant solution that contains both chlorine (HOCl, OCl−) and reactive oxygen species (e.g., ·OH, O3, H2O2, and ·O2−) capable of inactivating pathogens. One onsite generator of ECO is the Smart Electrochlorinator 200 (SE-200, Cascade Designs, Inc.). In a laboratory study, we assessed ECO surface disinfection efficacy for two gastrointestinal virus surrogates: bacteriophage MS2 and murine norovirus MNV-1. We quantified both infectivity and nucleic acid inactivation using culture-dependent and independent assays. At free available chlorine concentrations of 2,500 ppm and a contact time of 30 s, ECO inactivation of infective MS2 bacteriophage exceeded 7 log10 compared to MNV-1 disinfection of approximately 2 log10. Genomic RNA inactivation was less than infective virus inactivation: MS2 RNA inactivation was approximately 5 log10 compared to MNV-1 RNA inactivation of approximately 1.5 log10. The results are similar to inactivation efficacy of household bleach when used at similar free available chlorine concentrations. Our work demonstrates the potential of ECO solutions, generated onsite, to be used for surface disinfection.


Similar content being viewed by others
References
Abad, F. X., Pinto, R. M., & Bosch, A. (1994). Survival of enteric viruses on environmental fomites. Applied and Environmental Microbiology, 60(10), 3704–3710.
Abad, F. X., Pintó, R. M., & Bosch, A. (1997). Disinfection of human enteric viruses on fomites. FEMS Microbiology Letters, 156(1), 107–111.
Abbaszadegan, M., Mayer, B. K., Ryu, H., & Nwachuku, N. (2007). Efficacy of removal of CCL viruses under enhanced coagulation conditions. Environmental Science and Technology, 41(3), 971–977.
Aghababian, R. V., & Teuscher, J. (1992). Infectious diseases following major disasters. Annals of Emergency Medicine, 21(4), 362–367.
Bae, J., & Schwab, K. J. (2008). Evaluation of murine Norovirus, feline calicivirus, poliovirus, and MS2 as surrogates for human Norovirus in a model of viral persistence in surface water and groundwater. Applied and Environmental Microbiology, 74(2), 477–484.
Barker, J., Vipond, I., & Bloomfield, S. (2004). Effects of cleaning and disinfection in reducing the spread of norovirus contamination via environmental surfaces. Journal of Hospital Infection, 58(1), 42–49.
Block, S. S. (2001). Disinfection, sterilization and preservation. Lawrence: Wolters Kluwer Health.
Bright, K. R., Boone, S. A., & Gerba, C. P. (2009). Occurrence of bacteria and viruses on elementary classroom surfaces and the potential role of classroom hygiene in the spread of infectious diseases. Journal of School Nursing, 26(1), 33–41.
Cromeans, T. L., Kahler, A. M., & Hill, V. R. (2010). Inactivation of adenoviruses, enteroviruses, and murine norovirus in water by free chlorine and monochloramine. Applied and Environmental Microbiology, 76(4), 1028–1033.
Dalling, J. (2004). A review of environmental contamination during outbreaks of Norwalk-like virus. British Journal of Infection Control, 5(2), 9–13.
Dietrich, J. P., Başağaoğlu, H., Loge, F. J., & Ginn, T. R. (2003). Preliminary assessment of transport processes influencing the penetration of chlorine into wastewater particles and the subsequent inactivation of particle-associated organisms. Water Research, 37(1), 139–149.
D’Souza, D. H., & Su, X. (2010). Efficacy of chemical treatments against murine norovirus, feline calicivirus, and MS2 bacteriophage. Foodborne Pathogens and Disease, 7(3), 319–326.
Duizer, E., Schwab, K. J., Neill, F. H., Atmar, R. L., Koopmans, M. P., & Estes, M. K. (2004). Laboratory efforts to cultivate noroviruses. Journal of General Virology, 85(1), 79–87.
Gelinas, P., & Goulet, J. (1983). Neutralization of the activity of eight disinfectants by organic matter. Journal of Applied Microbiology, 54(2), 243–247.
Ghebremichael, K., Muchelemba, E., Petrusevski, B., & Amy, G. (2011). Electrochemically activated water as an alternative to chlorine for decentralized disinfection. Journal of Water Supply: Research and Technology—Aqua, 60(4), 210–218.
Gordon, G., Bolden, R., & Emmert, G. (2002). Measuring oxidant species in electrolyzed salt brine solutions. Journal American Water Works Association, 94(10), 111–120.
Jeong, J., Kim, J. Y., & Yoon, J. (2006). The role of reactive oxygen species in the electrochemical inactivation of microorganisms. Environmental Science and Technology, 40(19), 6117–6122.
Krilov, L. R., Barone, S. R., Mandel, F. S., Cusack, T. M., Gaber, D. J., & Rubino, J. R. (1996). Impact of an infection control program in a specialized preschool. American Journal of Infection Control, 24(3), 167–173.
LeChevallier, M. W., Hassenauer, T. S., Camper, A. K., & McFeters, G. A. (1984). Disinfection of bacteria attached to granular activated carbon. Applied and Environmental Microbiology, 48(5), 918–923.
Len, S.-V., Hung, Y.-C., Chung, D., Anderson, J. L., Erickson, M. C., & Morita, K. (2002). Effects of storage conditions and pH on chlorine loss in electrolyzed oxidizing (EO) water. Journal of Agricultural and Food Chemistry, 50(1), 209–212.
Li, J. W., Xin, Z. T., Wang, X. W., Zheng, J. L., & Chao, F. H. (2004). Mechanisms of inactivation of hepatitis A virus in water by chlorine dioxide. Water Research, 38(6), 1514–1519.
Martínez-Huitle, C. A., & Brillas, E. (2008). Electrochemical alternatives for drinking water disinfection. Angewandte Chemie International Edition, 47(11), 1998–2005.
Mattle, M. J., Crouzy, B., Brennecke, M. R., Wigginton, K., Perona, P., & Kohn, T. (2011). Impact of virus aggregation on inactivation by peracetic acid and implications for other disinfectants. Environmental Science and Technology, 45(18), 7710–7717.
Nuanualsuwan, S., & Cliver, D. O. (2003). Capsid functions of inactivated human picornaviruses and feline calicivirus. Applied and Environmental Microbiology, 69(1), 350–357.
O’Connell, K. P., Bucher, J. R., Anderson, P. E., Cao, C. J., Khan, A. S., Gostomski, M. V., et al. (2006). Real-time fluorogenic reverse transcription-PCR assays for detection of bacteriophage MS2. Applied and Environmental Microbiology, 72(1), 478.
Patel, M. M., Hall, A. J., Vinjé, J., & Parashar, U. D. (2009). Noroviruses: a comprehensive review. Journal of clinical virology: The official publication of the Pan American Society for Clinical Virology, 44(1), 1.
Pecson, B. M., Martin, L. V., & Kohn, T. (2009). Quantitative PCR for determining the infectivity of bacteriophage MS2 upon inactivation by heat, UVB and singlet oxygen: advantages and limitations of an enzymatic treatment to reduce false-positive results. Applied and Environmental Microbiology, 75(17), 5544–5554.
Pickering, A. J., Julian, T. R., Marks, S. J., Mattioli, M. C., Boehm, A. B., Schwab, K. J., et al. (2012). Fecal contamination and diarrheal pathogens on surfaces and soil show spatial heterogeneity within Tanzanian households and no association with improved sanitation. Environmental Science and Technology, 46(11), 5736.
Reckhow, D. A., Singer, P. C., & Malcolm, R. L. (1990). Chlorination of humic materials: Byproduct formation and chemical interpretations. Environmental Science and Technology, 24(11), 1655–1664.
Rivera, S. B., Zuback, R., & Goodwin, W. P. (2009). Improving access to safe water through technology and informed design. Proceedings of the Water Environment Federation, 2009(1), 565–574.
Sanglay, G.C. (2012). Inactivation and mechanism of electron beam irradiation and sodium hypochlorite sanitizers against a human norovirus surrogate (PhD thesis). Ohio State University, Columbus, OH.
Sinclair, R. G., & Gerba, C. P. (2011). Microbial contamination in kitchens and bathrooms of rural Cambodian village households. Letters in Applied Microbiology, 52(2), 144–149.
Son, H., Cho, M., Kim, J., Oh, B., Chung, H., & Yoon, J. (2005). Enhanced disinfection efficiency of mechanically mixed oxidants with free chlorine. Water Research, 39(4), 721–727.
Teunis, P. F. M., Moe, C. L., & Liu, P. (2008). Norwalk virus: How infectious is it? Journal of Medical Virology, 80(8), 1468–1476.
Tung, G., Macinga, D., Arbogast, J., & Jaykus, L.-A. (2013a). Efficacy of commonly used disinfectants for inactivation of human noroviruses and their surrogates. Journal of Food Protection, 76(7), 1210–1217.
Tung, G., Macinga, D., Arbogast, J., & Jaykus, L.-A. (2013b). Efficacy of commonly used disinfectants for inactivation of human noroviruses and their surrogates. Journal of Food Protection®, 76(7), 1210–1217.
Urakami, H., Ikarashi, K., Okamoto, K., Abe, Y., Ikarashi, T., Kono, T., et al. (2007). Chlorine sensitivity of feline calicivirus, a norovirus surrogate. Applied and Environmental Microbiology, 73(17), 5679–5682.
USEPA. (2001). EPA method 1602: Male-specific (F+) and somatic coliphage in water by single agar layer (SAL) procedure. Washington, DC: Environmental Protection Agency.
Venczel, L. V., Arrowood, M., Hurd, M., & Sobsey, M. D. (1997). Inactivation of Cryptosporidium parvum oocysts and Clostridium perfringens spores by a mixed-oxidant disinfectant and by free chlorine. Applied and Environmental Microbiology, 63(4), 1598–1601.
Venczel, L., Likirdopulos, C., Robinson, C., & Sobsey, M. (2004). Inactivation of enteric microbes in water by electro-chemical oxidant from brine (NaCl) and free chlorine. Water Science and Technology, 50(1), 141–146.
Ward, R., Bernstein, D., Knowlton, D., Sherwood, J., Young, E., Cusack, T., et al. (1991). Prevention of surface-to-human transmission of rotaviruses by treatment with disinfectant spray. Journal of Clinical Microbiology, 29(9), 1991–1996.
Wick, H., & Charles, P. E. M. (1999). Characterization of purified MS2 bacteriophage by the physical counting methodology used in the integrated virus detection system (IVDS). Toxicology Mechanisms and Methods, 9(4), 245–252.
Wigginton, K. R., Pecson, B. M., Sigstam, Tr, Bosshard, F., & Kohn, T. (2012). Virus inactivation mechanisms: Impact of disinfectants on virus function and structural integrity. Environmental Science and Technology, 46(21), 12069–12078.
Wilmouth, R., Forsyth, J., & Meschke, J. S. (2011). Evaluation of the implementation (Pilot) of a small community electrochlorination device-smart electrochlorinator (SE200)-at a field site in a peri-urban community (Kisumu, Kenya). Proceedings of the Water Environment Federation, 2011(3), 253–273.
Winward, G. P., Avery, L. M., Stephenson, T., & Jefferson, B. (2008). Chlorine disinfection of grey water for reuse: Effect of organics and particles. Water Research, 42(1–2), 483–491.
Wobus, C. E., Karst, S. M., Thackray, L. B., Chang, K.-O., Sosnovtsev, S. V., Belliot, G., et al. (2004). Replication of norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biology, 2(12), e432.
Wobus, C. E., Thackray, L. B., & Virgin, H. W. (2006). Murine norovirus: A model system to study norovirus biology and pathogenesis. Journal of Virology, 80(11), 5104–5112.
Young, D. C., & Sharp, D. G. (1985). Virion conformational forms and the complex inactivation kinetics of echovirus by chlorine in water. Applied and Environmental Microbiology, 49(2), 359–364.
Acknowledgments
The work was supported by Cascade Designs, Inc., the Osprey Foundation of Maryland, Inc., and the Johns Hopkins University Global Water Program. Cascade Designs, Inc. provided financial resources and input into the experimental design but was not otherwise involved in the study. We would like to thank Rebecca Pinekenstein for laboratory assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Julian, T.R., Trumble, J.M. & Schwab, K.J. Evaluating Efficacy of Field-Generated Electrochemical Oxidants on Disinfection of Fomites Using Bacteriophage MS2 and Mouse Norovirus MNV-1 as Pathogenic Virus Surrogates. Food Environ Virol 6, 145–155 (2014). https://doi.org/10.1007/s12560-014-9136-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12560-014-9136-6