Abstract
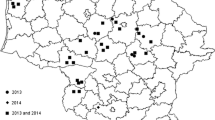
Isolates of the Fusarium graminearum species complex (FGSC, n = 446) were collected from wheat spikes from northern and western regions of Iran with a history of Fusarium head blight (FHB) occurrences. The trichothecene mycotoxin genotypes/chemotypes, the associated phylogenetic species, and geographical distribution of these isolates were analyzed. Two phylogenetic species, Fusarium asiaticum and F. graminearum, were identified and were found to belong to sequence characterized amplified region (SCAR) groups V and I. Isolates from F. asiaticum species lineage 6 were within SCAR group V, whereas F. graminearum species lineage 7 were of SCAR group I. Of the 446 isolates assayed, 274 were F. asiaticum species predominantly of the nivalenol (NIV) genotype, while other isolates were either deoxynivalenol (DON) plus 3-acetyldeoxynivalenol (3-AcDON) or DON plus 15-acetyldeoxynivalenol (15-AcDON) genotype. Based on Tri7 gene sequences, a new subpopulation of 15-AcDON producers was observed among F. asiaticum strains in which 11-bp repeats were absent in the Tri7 sequences. The trichothecene chemotype was confirmed and quantified by high-performance liquid chromatography (HPLC) in 46 FGSC isolates. Isolates produced NIV (33.4–108.2 μg/g) and DON (64.7–473.6 μg/g) plus either 3-AcDON (51.4–142.4 μg/g) or 15-AcDON (24.1–99.3 μg/g). Among FGSC isolates, F. asiaticum produced the highest levels of trichothecenes. Using BIOCLIM based on the climate data of 20-year during 1994–2014, modelling geographical distribution of FGSC showed that F. asiaticum was restricted to warmer and humid areas with a median value of mean annual temperature of about 17.5 °C and annual rainfall of 658 mm, respectively (P < 0.05). In contrast, F. graminearum (only 15-AcDON producers) was restricted to cooler and drier areas, with a median value of the mean annual temperature of 14.4 °C and an annual rainfall of 384 mm, respectively (P < 0.05). Based on climate parameters at anthesis, the recorded distribution of F. graminearum and F. asiaticum was similar to that based on BIOCLIM parameters. Therefore, geographic differences on the wheat-growing areas in Iran have had a significant effect on distribution of FGSC and their trichothecene chemotypes.





Similar content being viewed by others
References
Abedi-Tizaki M, Sabbagh SK (2012) Morphological and molecular identification of Fusarium head blight isolates from wheat in North of Iran. Aust J Crop Sci 6:1356–1361
Abedi-Tizaki M, Sabbagh SK (2013) Detection of 3-acetyldeoxynivalenol, 15 acetyldeoxynivalenol and nivalenol-chemotypes of Fusarium graminearum from Iran using specific PCR assays. Plant Knowledge J 2:38–42
Abedi-Tizaki M, Zafari DM (2015) Natural occurrence of deoxynivalenol and its derivatives in wheat in north of Iran. J Plant Pathol 97:1–7
Abedi-Tizaki M, Sabbagh SK, Mazaheri-Naeini M, Sepehrikia S (2013) Chemotyping of Fusarium graminearum using Tri13 trichothecene biosynthetic gene. J Crop Prot 2:487–500
Astolfi P, dosSantos J, Schneider L, Gomes LB, Silva CN, Tessmann DJ, Del Ponte EM (2011) Molecular survey of trichothecene genotypes of Fusarium graminearum species complex from barley in southern Brazil. Int J Food Microbiol 148:197–201
Backhouse D (2014) Global distribution of Fusarium graminearum, F. asiaticum and F. boothii from wheat in relation to climate. Eur J Plant Pathol 139:161–173
Backhouse D, Burgess LW (2002) Climatic analysis of the distribution of Fusarium graminearum, F. pseudograminearum and F. culmorum on cereals in Australia. Australas Plant Pathol 31:321–327
Bennett JW, Klich M (2003) Mycotoxins. Clin Microbiol Rev 16:497–516
Buerstmayr M, Huber K, Heckmann J, Steiner B, Nelson JC, Buerstmayr H (2012) Mapping of QTL for Fusarium head blight resistance and morphological and developmental traits in three backcross populations derived from Triticum dicoccum x Triticum durum. Theor Appl Genet 125:1751–1765
Carter JP, Razanoor HN, Desjardins AE, Nicholson P (2000) Variation in Fusarium graminearum isolates from Nepal associated with their host of origin. Plant Pathol 49:1–10
Chandler EA, Simpson DR, Thomsett MA, Nicholson P (2003) Development of PCR assays to Tri7 and Tri13 trichothecene biosynthetic genes and characterisation of chemotypes of Fusarium graminearum, Fusarium culmorum and Fusarium cerealis. Physiol Mol Plant Pathol 62:355–367
Davari M, Wei SH, Babay-Ahari A, Arzanlou M, Waalwijk C, Lee TAJ, Zare R, Gerrits van den Ende AHG, de Hoog GS, van Diepeningen AD (2013) Geographic differences in trichothecene chemotypes of Fusarium graminearum in the Northwest and North of Iran. World Mycotoxin J 6:137–150
De Wolf ED, Madden LV, Lipps PE (2003) Risk assessment models for wheat Fusarium head blight epidemics based on within-season data. Phytopathology 93:428–435
Desjardins AE (2006) Fusarium mycotoxins. Chemistry, Genetics, and Biology, American Phytopathological Society Press, St Paul
Hijmans RJ, Graham CH (2006) The ability of climate envelope models to predict the effect of climate change on species distributions. Glob Chang Biol 12:2272–2281
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
IARC, International Agency for Research on Cancer Working Group on the Evaluation of Carcinogenic risks to Humans (1993) Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins. Monographs on the evaluation of carcinogenic risks to humans, vol. 56
Jennings P, Coates ME, Turner JA, Chandler EA, Nicholson P (2004) Determination of deoxynivalenol and nivalenol chemotypes of Fusarium culmorum isolates from England and Wales by PCR assays. Plant Pathol 53:182–190
Jeon JJ, Kim H, Kim HS, Zeller KA, Lee T, Yun SH, Bowden RL, Leslie JF, Lee YW (2003) Genetic diversity of Fusarium graminearum from maize in Korea. Fungal Genet Newsl 50(Suppl):438
Ji L, Cao KL, Hu J, Wang TS (2007) Determination of deoxynivalenol and nivalenol chemotypes of Fusarium graminearum isolates from China by PCR assay. J Phytopathol 155:505–512
Kimura M, Tokai T, O'Donnell K, Ward TJ, Fujimura M, Hamamoto H, Shibata T, Yamaguchi I (2003) The trichothecene biosynthesis gene cluster of Fusarium graminearum F15 contains a limited number of essential pathway genes and expressed non-essential genes. FEBS Lett 539:105–110
Kimura M, Tokai T, Takahashi-Ando N, Ohsato S, Fujimura M (2007) Molecular and genetic studies of Fusarium trichothecene biosynthesis; pathways, genes and evolution. Biosci Biotechnol Biochem 71:2105–2123
Kriss AB, Paul PA, Xu X, Nicholson P, Doohan FM, Hornok L, Reitini A, Edwards SG, Madden LV (2012) Quantification of the relationship between the environment and Fusarium head blight, Fusarium pathogen density, and mycotoxins in winter wheat in Europe. Eur J Plant Pathol 133:975–993
Lee T, Oh D, Kim H, Kim HS, Lee J, Kim YH, Yun SH, Lee YW (2001) Identification of deoxynivalenoland nivalenol-producing chemotypes of Gibberella zeae by using PCR. Appl Environ Microbiol 67:2966–2972
van der Lee T, Zhang H, van Diepeningen A, Waalwijk C (2015) Biogeography of Fusarium graminearum species complex and chemotypes. Food Addit Contam Part A 32(4):453–460
Li HP, Wu AB, Zhao CS, Scholten O, Loffler H, Liao YC (2005) Development of a generic PCR detection of deoxynivalenol and nivalenol chemotypes of Fusarium graminearum. FEMS Microbiol Lett 243:505–511
Mirabolfathy M, Karami-Osboo R (2013) Deoxynivalenol and DON -producing Fusarium graminearum isolates in wheat and barley crops in north and northwest areas of Iran. Iran J Plant Pathol 48:197–210
Nelson PE, Toussoun TA, Marasas WFO (1983) Fusarium species: an illustrated manual for identification. The Pennsylvania State University Press, University Park
Nicholson P, Rezanoor HN, Simpson DR, Joyce D (1997) Differentiation and quantification of the cereal eyespot fungi Tapesia yallundae and Tapesia acuformis using a PCR assay. Plant Pathol 46:842–856
O’Donnell K, Kistler HC, Tacke BK, Casper HH (2000) Gene genealogies reveal global phylogeographic structure and reproductive isolation among lineages of Fusarium graminearum, the fungus causing wheat scab. Proceedings of the National Academy of Sciences, USA 97:7905–7910
O’Donnell K, Ward TJ, Geiser DM, Kistler HC, Aoki T (2004) Genealogical concordance between the mating type locus and seven other nuclear genes supports formal recognition of nine phylogenetically distinct species within the Fusarium graminearum clade. Fungal Genet Biol 41:600–623
O’Donnell K, Ward TJ, Aberra D, Kistler HC, Aoki T, Orwig N, Kimura M, Bjornstad A, Klemsdal SS (2008) Multilocus genotyping and molecular phylogenetics resolve a novel head blight pathogen within the Fusarium graminearum species complex from Ethiopia. Fungal Genet Biol 45:1514–1522
Oliver RE, Cai X, Friesen TL, Halley S, Stack RW, Xu SS (2008) Evaluation of Fusarium head blight resistance in tetraploid wheat (Triticum turgidum L.) Crop Sci 48:213–222
Parikka P, Hakala K, Tiilikkala K (2012) Expected shifts in Fusarium species’ composition on cereal grain in Northern Europe due to climatic change. Food Addit Contam Part A 29:1543–1555
Pasquali M, Migheli Q (2014) Genetic approaches to chemotype determination in type B-trichothecene producing Fusaria. Int J Food Microbiol 189:164–182
Qu B, Li HP, Zhang JB, Huang T, Carter J, Liao YC, Nicholson P (2008) Comparison of genetic diversity and pathogenicity of Fusarium head blight pathogens from China and Europe by SSCP and seedling assays on wheat. Plant Pathol 57:642–651
Ramirez ML, Reynoso MM, Farnochi MC, Torres AM, Leslie JF, Chulze SN (2007) Population genetic structure of Gibberella zeae isolated from wheat in Argentina. Food Addit Contam 24:1115–1120
Reynoso MM, Ramirez ML, Torres AM, Chulze SN (2011) Trichothecene genotypes and chemotypes in Fusarium graminearum strains isolated from wheat in Argentina. Int J Food Microbiol 145:444–448
Sarver BA, Ward TJ, Gale LR, Broz K, Kistler HC, Aoki T, Nicholson P, Carter J, O’Donnell K (2011) Novel Fusarium head blight pathogens from Nepal and Louisiana revealed by multilocus genealogical concordance. Fungal Genet Biol 48:1096–1107
Starkey DE, Ward TJ, Aoki T, Gale LR, Kistler HC, Geiser DM, Suga H, Tòth B, Varga J, O’Donnell K (2007) Global molecular surveillance reveals novel Fusarium head blight species and trichothecene toxin diversity. Fungal Genet Biol 44:1191–1204
Suga H, Karugia GW, Ward T, Gale LR, Tomimura K, Nakajima T, Kageyama K, Hyakumachi M (2005) Development of a PCR-RFLP-based identification system for Fusarium asiaticum and genetic characterization of western Japanese isolates. Fungal Genet Newsl 52(Suppl):118
Suga H, Karugia GW, Ward T, Gale LR, Tomimura K, Nakajima T, Miyasaka A, Koizumi S, Kageyama K, Hyakumachi M (2008) Molecular characterization of the Fusarium graminearum species complex in Japan. Phytopathology 98:159–166
Umpierrez-Failache M, Garmendia G, Pereyra S, Rodriquez-Haralambides A, Ward TJ, Vero S (2013) Regional differences in species composition and toxigenic potential among Fusarium head blight isolates from Uruguay indicate a risk of nivalenol contamination in new wheat production areas. Int J Food Microbiol 166:135–140
Ward TJ, Bielawski JP, Kistler HC, Sullivan E, O’Donnell K (2002) Ancestral polymorphism and adaptive evolution in the trichothecene gene cluster of phytopathogenic Fusarium. Proceedings of the National Academy of Sciences of the United States of America 99, 9278–9283
Windels CE (2000) Economic and social impacts of Fusarium head blight: changing farms and rural communities in the Northern Great Plains. Phytopathology 90:17–21
Yang L, van der Lee T, Yang X, Yu D, Waalwijk C (2008) Fusarium populations on Chinese barley show a dramatic gradient in mycotoxin profiles. Phytopathology 98:719–727
Zeller KA, Bowden RL, Leslie JF (2003) Diversity of epidemic populations of Gibberella zeae from small quadrats in Kansas and North Dakota. Phytopathology 93:874–880
Zhang JB, Li HP, Dang FJ, Qu B, Xu YB, Zhao CS, Liao YC (2007) Determination of the trichothecene mycotoxin chemotypes and associated geographical distribution and phylogenetic species of the Fusarium graminearum clade from China. Mycol Res 111:967–975
Zhang H, van der Lee T, Waalwijk C, Chen W, Xu J, Xu J, Zhang Y, Feng J (2012) Population analysis of the Fusarium graminearum species complex from wheat in China show a shift to more aggressive isolates. PLoS One 7(2):e31722
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None
Rights and permissions
About this article
Cite this article
Abedi-Tizaki, M., Zafari, D. Geographic distribution of phylogenetic species of the Fusarium graminearum species complex and their 8-ketotrichothecene chemotypes on wheat spikes in Iran. Mycotoxin Res 33, 245–259 (2017). https://doi.org/10.1007/s12550-017-0283-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12550-017-0283-0