Abstract
Historical evidence suggests that social status played a major role in all aspects of society in eighteenth–nineteenth century England. We present an insight into how socioeconomic status affected the dietary habits of two post-medieval urban populations from Greater Manchester, northwest England. Stable carbon (δ13C) and nitrogen (δ15N) isotope ratios were measured in humans from Cross Street Unitarian Chapel (middle class: n = 90) in Manchester city centre and Chapel Street, Hazel Grove (lower status with few middle-class individuals: n = 34). A large sample of 111 faunal remains from Cross Street (n = 37) and Norton Priory, Cheshire (n = 74), provide an animal baseline, dramatically expanding the post-medieval animal isotopic dataset for England. Sheep from Norton Priory show high δ15N isotope values indicative of saltmarsh grazing. Results for human populations revealed a mixed diet of plant and animal protein from C3 terrestrial environments with some potential contribution of aquatic protein. Significant differences revealed between the two populations indicate unequal access to food by status. Intra-population variation at Hazel Grove suggests dietary distinctions by age and sex. Non-adults consumed diets poor in high trophic level protein, whereas adult males consumed greater amounts of animal products. Conversely, the data suggests that at the wealthier Cross Street, there was greater access to high trophic level protein by all. Comparisons between the Manchester populations and those from similar socioeconomic classes from the Midlands and southern England, including London, reveal a bioarchaeological picture of dietary diversity and differential access to resources which impact significantly on well-being during this tumultuous period of industrial England.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Industrial Revolution of eighteenth–nineteenth century England was one of the most dynamic periods in British history (O’Brien and Quinault 1993). This era was a time of great population growth, scientific invention, and rapid urbanisation. Prior to the seventeenth century, a self-sustaining ecosystem existed, whereby food was largely derived from a small-scale peasant type of agriculture. However, improvements in agriculture in the seventeenth century meant that there was greater farm produce achieved with fewer workers (O’Brien and Quinault 1993; Burnett 2005). New machinery also led to rapid changes in the manufacturing industry, which subsequently increased the movement of the rural poor to developing towns and cities for work (O’Brien and Quinault 1993; Burnett 2005). Consequently, populations grew rapidly in these transforming towns and urban centres (O’Brien and Quinault 1993).
Manchester was one of the urban centres that transformed during this period, becoming Britain’s most important industrial city after London after 1750 (Glinert 2009). Historical records indicate that in the second half of the eighteenth century, a number of factors transformed the city into a major industrial conurbation, with some naming it the ‘Western world’s first industrialised city’ (Douglas et al. 2002, 236). The combination of the number of coal mines, the construction of the Bridgewater canal to supply coal to cotton mills and new technological innovations in the long-established wool and linen textile industry all contributed to the major industrialisation of the city. Manchester also pioneered the development of the world’s first inter-city passenger and goods railway to Liverpool’s ports, which increased trade without restrictions. This industrial dynamism led to an extensive population growth from nearly 77,000 in 1801 to 316,000 in 1851, with 54% coming from elsewhere in the British Isles and 1% from overseas (Douglas et al. 2002, 237). Extensive population growth in Manchester increased the local demand for food, and due to improved communications, it meant that food in the city was sourced from increasingly distant parts of Britain and even other countries (Chaloner 1959; Scola 1992). As Manchester expanded, the city developed well-demarcated zones that divided people into classes: the working class, which included low-skilled factory workers, and the middle/upper class, who owned most of the means of production (Scola 1992, 9). Expanding urbanisation placed heavy demands on food supplies and availability and access to food became wage dependent (Engels 1952), contributing to the creation of distinct food consumption patterns based on social classes. In addition to class, social groupings such as age and sex within these communities also governed dietary habits (Scola 1992).
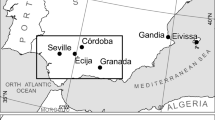
In contrast to earlier periods in Britain, biomolecular dietary research in the post-medieval period received little attention until relatively recently. However, since archaeological companies took over the excavation of post-medieval burials disturbed by urban development from crypt clearance companies in the early 2000s, a considerable number of skeletal remains have been recorded archaeologically (Powers et al. 2013). The availability of archaeological skeletal populations from this period coupled with the opportunities presented by a rich historical resource to draw upon (e.g. Lightfoot et al. (2020)) means that the complexities of post-medieval diets are increasingly being explored using scientific methods. Within the last few years, isotope analyses on post-medieval populations in Britain have grown in number, e.g. Richards (2006), Trickett (2006), Nitsch et al. (2010), Nitsch et al. (2011), Roberts et al. (2012), Beaumont (2013), Beaumont et al. (2013a), Beaumont et al. (2013b), Brown and Alexander (2016), Bleasdale et al. (2019) and Dhaliwal et al. (2020). These studies have demonstrated the ways in which stable isotope data can enhance the deciphering of dietary habits in post-medieval populations. However, these studies typically relate to cemeteries in London or cities in the Midlands primarily because of the number of excavations as a result of developmental proposals. Over 5000 post-medieval burials were excavated between 2008 and 2013 in London alone (Powers et al. 2013, 126), a number greatly increased by the recent HS2 and Cross Rail projects. This study, in contrast, focuses on two urban cemeteries from what is now Greater Manchester in northwest England (Fig. 1). The two populations both date to the eighteenth and nineteenth centuries and derive from different social groups; Cross Street Unitarian Chapel represents mostly middle-class individuals with a few lower-class individuals (Keefe and Holst 2017), whereas Chapel Street, Hazel Grove, comprises a mixed middle/low-class population, with a greater proportion of the poorer classes (Newman and Holst 2016). It is worth noting that there is a small number of individuals from Hazel Grove whose cranial morphology could be suggestive of African/mixed ancestry (Newman and Holst 2016) (see below). This research aims to provide new insights into the diet of these two populations through carbon (δ13C) and nitrogen (δ15N) isotope analysis of human and animal bone collagen with a focus on possible age, status, ancestry and sex variation in diet.
Site background
Cross Street Unitarian Chapel
Archaeological excavations at Cross Street Unitarian Chapel, Manchester, were commissioned by Transport for Greater Manchester (project code TFGM3/2162) and conducted by CFA Archaeology Ltd between September 2014 and November 2015 in advance of the construction of the Second City Crossing tramway along Cross Street in the northern half of Manchester city centre (CFA Archaeology Ltd 2017a, 5–6). The Unitarian Chapel was originally erected in 1694 by Reverend Henry Newcome to accommodate his growing congregation, and the cemetery was in use for just over 130 years, with the earliest grave (no surviving skeleton) dating to 1720 and the latest to 1852. Like many other non-conformist groups, the chapel was initially attended by the poorer classes; however, with time, the chapel recruited more prosperous members from the manufacturing classes or those who were artisans or merchants (Baker 1884). People interred at the Cross Street burial ground came from wealthy streets in Manchester such as Ardwick, Mosley Street, Brazennose Street, Holme Street and Princess Street (CFA Archaeology Ltd 2017b). Some of the members of the congregation who were buried at the chapel in the late eighteenth century include wealthy cotton merchants, such as Thomas and Richard Potter (Baker 1884, 95). The first Mayor of Manchester, Sir John Potter, a ‘very wealthy man’, according to the description given by Messinger (1985, 59), also worshipped in this chapel. Sir John Potter himself was not exhumed during the redevelopment of Cross Street but some individuals were excavated who had grave markers denoting the burials of members of the Potter family, for instance, Skeletons 2.31 and 2.34 (Elizabeth and Katherine Potter) who are part of this study. On the whole, the population are considered to be modest-living middle-class citizens. A total of 241 individual skeletons and 17,679 fragments of disarticulated bone were recovered. Among the individuals recovered at this site, 172 could be identified based on legible nameplates, coffins with studwork text, or legible gravestones, as well as documentary sources, 26 of whom were analysed in this study (CFA Archaeology Ltd 2017b, 5–6) (Table S1).
Osteological analysis carried out by York Osteoarchaeology Ltd on all 241 skeletons as well as on disarticulated bones recovered at this site revealed that the proportion of non-adults recovered (28%) was lower than expected from historical documents for this period. The London Bills of Mortality suggest that around 50% of the population died before the age of 20 years between the early eighteenth and mid-nineteenth centuries (Roberts and Cox 2003, 304). Among the sexed adults, there was a slightly higher proportion of males (51.1%) than females (48.9%) (Keefe and Holst 2017). In terms of osteological indicators of diet and nutrition, dental health at this site was generally poor; females showed high levels of caries, with 20.7% of their teeth affected compared to 18.5% of teeth from males. Antemortem tooth loss was observed for both males and females. Just over three-quarters of the adults with at least one observable tooth position (94/122, 77.0%) had experienced antemortem tooth loss, and 27.5% of their teeth had been lost antemortem. This is considered as consistent with a diet of refined sugars and processed carbohydrates. Conditions associated with lack of specific nutrients such as scurvy (lack of vitamin C which can be found in fresh fruit, vegetables, and marine fish) and rickets (lack of vitamin D, which is produced by the body during exposure to sunlight as well as other sources like oily fish, eggs, and milk) were observed in non-adults. The non-adult prevalence rates for scurvy and rickets in this population were 5.9% and 4.4%, respectively, relatively low when compared to contemporary urban populations (Keefe and Holst 2017). Children with these conditions also had evidence of higher rates of enamel defects than adults (Keefe and Holst 2017).
Excavations at this site also uncovered unstratified animal remains. Industrial services disturbed the burial ground from the nineteenth century onwards; therefore, these animal bones might be from a later date than the graveyard. However, in the absence of any other suitable animal material from the city itself, these remains were sampled for an animal baseline.
Chapel Street, Hazel Grove
In 2016, CFA Archaeology excavated the site of a former Wesleyan Chapel on Chapel Street, Hazel Grove, Greater Manchester. Hazel Grove is presently a suburb located within the Metropolitan Borough of Stockport, approximately 9 miles from Manchester city centre (Fig. 1). The Wesleyan Chapel dates to around 1785, and a Sunday school was in place between c1823 and 1912, and the cemetery was in use between 1794 and 1910. Most of the population living in Hazel Grove were those of the poor classes (Errington 2001; Newman and Holst 2016). Osteological analysis was carried out on 38 skeletons recovered from the southwest and northwest area behind the church by York Osteoarchaeology Ltd in 2016 (Newman and Holst 2016). The nameplates recovered during the excavations (Table S1) reveal that the excavated individuals were representative of the eighteenth and early nineteenth century members of the congregation (Newman and Holst 2016).
Only a small proportion of the adult burial population could be sexed, indicating the presence of six males and seven females. Half of the population was made up of non-adults, with a high proportion of infants (1 to 11 months, 52.6%) and young juveniles (1 to 6 years, 31.6%) which correspond with historical documentation that states that a large proportion of deaths occurred within the first 5 years of life during this time period (Cherryson et al. 2012).
Osteological analysis also revealed poor dental health at this site (Newman and Holst 2016). Females showed higher levels of caries than males, but there was disproportionality between sexes with 49.1% of their teeth affected compared to 7.2% of teeth from males. This was likely skewed by two female individuals that had a significant number of carious lesions (Newman and Holst 2016). Antemortem tooth loss was observed in both sexes. Just under three-quarters of the adults from Hazel Grove (11/15, 73.3%) had experienced antemortem tooth loss, and 22.64% of their teeth had been lost antemortem. Similar to Cross Street, poor dental health was consistent with a diet of refined sugars and processed carbohydrates. Palaeopathological analysis of the Hazel Grove population suggested that this group had suffered from periods of ill health. Six infants and young juveniles suffered from scurvy and rickets, with a prevalence rate of 15.8% (3/19) for both (Newman and Holst 2016). Enamel defects, cribra orbitalia, and residual rickets, all evidence for childhood stress, were also identified among adults.
Within the Hazel Grove population, four individuals tentatively exhibited cranial morphological traits as described by Byers (2010, 154–165) that could be suggestive of African/mixed ancestry (SK 1, SK 10, SK 14 and SK 21) (Newman and Holst 2016, 24–25). In addition, DNA test results revealed that two of the individuals (SK 1 and SK 10) were likely of mixed ancestry (Banken 2018).
Norton Priory Chester
To supplement the animal remains sampled from Cross Street, further animal bone was sampled from Norton Priory, Runcorn, Cheshire, approximately 27.7 miles from Manchester, which represented a broadly contemporaneous dataset from northwest England (Fig. 1). Excavations at Norton Priory were undertaken between 1970 and 1987 by the Runcorn Development Corporation and focussed on the buildings of Priory of St. Mary (1134–1536) and some of the areas occupied post-dissolution (Wright et al. 2017). Animal remains were sampled from the post-dissolution Period 2 between 1536 and c1730, representative of the suppression of the priory, and the Tudor and later occupation of Norton Hall, as well as Period 3 between c1730 and 1928, representative of the occupation of the Georgian Mansion (Wright et al. 2017), contemporaneous with the humans from Greater Manchester. Although the house was finally demolished in 1928, there are indications that the house became gradually less occupied by the late nineteenth century (Brown and Howard-Davis 2008), and animal remains found at this site are unlikely to date beyond this period. The majority of the samples came from a large midden, assigned to Period 2 (1536–c1730) which was located near the kitchens (Brown and Howard-Davis 2008). The rest of the faunal assemblage was recovered from undifferentiated topsoil deposits from landscaping and the demolition of the house in 1928 (Brown and Howard-Davis 2008; Wright et al. 2017). As a result, in this study, all the remains from Periods 2 and 3 were combined for analysis.
Diet in Manchester
Historic accounts of typical foods consumed by the eighteenth to nineteenth century Manchester population indicate the diet was primarily based on C3 plant foods such as rye, wheat and oats in the form of bread and porridge (Chaloner 1959; Scola 1992). The Corn Laws of 1815, however, had a disastrous effect on grain prices which particularly affected the poorer classes who were heavily dependent on bread as a staple food. As a result, people turned to potatoes as a food source (Pickering and Tyrell 2000). In addition to C3 foods, minor contributions from C4 resources such as cane sugar and maize formed part of the diet in Manchester (Scola 1992). Cane sugar was mostly afforded by the rich until after the abolition of the Sugar Tax in 1874, when it became available to all classes (Walvin 2017), and maize had been provided to the Irish who had migrated to England as relief food during the Great Irish Famine of 1845 to 1852 (Ó’Gráda 1989).
The improvements of railways and steamships provided faster, cheaper and higher carrying capacity of livestock and meat into and within England and also gave farmers opportunities to sell their produce in larger urban areas like Manchester (Scola 1992; Schwartz 2010). Meat and fish were consumed especially by the middle and upper classes (Chaloner 1959; Scola 1992). Meat consumed consisted of veal, pork, sheep, poultry and beef. John Holt’s 1794 survey of agriculture in Lancashire revealed that except for veal and pork, very little of the meat consumed within the county was from local produce (Holt 1794). The poor could rarely afford beef, mutton and veal as they were expensive luxuries (Booth 1977; Scola 1992). Pork and poultry, including wildfowl of various kinds, however, were available in the city and were potentially commonly consumed by all (Chaloner 1959). Sheep and cattle were typically brought to the city from North Wales, the Welsh borders, Lincolnshire and the neighbouring counties (Redhead et al. 1792; Marshall 1818; Scola 1992). Furthermore, after the 1759 abolishment of the Cattle Acts which previously banned the importation of livestock from Ireland and the end of the Napoleonic Wars (1793–1815), the importation of cattle from Ireland into the city commenced (Blackman 1975) to the extent that by the mid-nineteenth century; about 80 to 90% of cattle were sourced either directly from Ireland or indirectly from Ireland via Liverpool (Scola 1992, 47). Although Manchester is inland, it received marine resources such as cod, lobsters and turbot from the Yorkshire coast and sole from Lancashire (Chaloner 1959, 44). These were, again, mainly afforded by the middle and upper classes. However, the poor could often afford mackerel and cured herring from the Isle of Man and the east coast herring fleets from Scotland, mostly in times of glut (Aikin 1785; Chaloner 1959, 44). The exact quantity of freshwater fish available for consumption is unknown, but it has been indicated that farming of freshwater fish was limited and had gone into decline from the time of the Reformation in the sixteenth century (North 1714). Other sources of food included dairy products such as milk, cheese and butter, fruits and vegetables which were mostly afforded by the wealthy (Scola 1992).
Dietary isotopes
The principles of using stable isotope analysis of carbon and nitrogen to quantify dietary practices of ancient populations are well established (Ambrose 1990; Tykot et al. 1996; Schwarcz and Schoeninger 2012; Szpak et al. 2017). Body tissues such as bones, used in this study, incorporate stable isotopes of carbon (δ13C) and nitrogen (δ15N) from foods consumed by individuals during their lifetime, and these isotopic signatures are retained after death (Schwarcz and Schoeninger 2012). In palaeodietary studies, changes in the ratio of the heavier to the lighter isotope are expressed in delta notation (δ) in parts per thousand (per mil or ‰), relative to an internationally agreed sample material (Roberts et al. 2018). Due to turnover, bone tissues represent an individual’s average diet over 10 years prior to death, or longer, depending on the skeletal element (Cox and Sealy 1997; Hedges et al. 2007). Bone collagen δ15N isotope values provide information on the main protein sources of the diet. Bone collagen δ13C isotope values also represent the protein sources in the diet in the majority although about 25% of bone collagen (non-essential amino acids) can be synthesised from lipids and carbohydrates (Fernandes et al. 2012).
The δ13C value of consumers is primarily affected by the photosynthetic pathway (C3 or C4) of plants such that C3 plants, such as wheat and barley, have lower δ13C isotope values than those of C4 plants, such as maize and sugarcane (Schwarcz and Schoeninger 2012). In addition to being valuable in distinguishing between C3 and C4 plant intake, δ13C isotope values can be utilised in differentiating marine and terrestrial (C3) protein diets, where the δ13C isotope values of the baseline of the former tend to be ~8‰ higher than that of the latter (Chisholm et al. 1982; Mays 1997). Most C4 plants are not indigenous to Britain but as already indicated, cane sugar and maize contributed to the diet in the Greater Manchester area in the eighteenth to nineteenth century (Barker et al. 1970; Scola 1992). Nitrogen isotope ratios provide information about the consumer’s trophic position in the food chain and differentiate terrestrial and marine food contributions to the consumer’s diet (Schoeninger et al. 1983; Minagawa and Wada 1984). It has also been established that δ15N isotope values can be elevated due to nutritional stress (Hobson et al. 1993; Fuller et al. 2004). Furthermore, δ15N values of plants can be increased through anthropogenic manuring input which in turn elevates consumers’ δ15N values (Hoefs 2015).
Materials and methods
Materials
In total, 54 adults and 36 non-adults from Cross Street were sampled. Of these, 27 were females, 25 were males and 38 were of unknown sex of which all but 3 were non-adults. In addition, we selected a total of 20 adults and 14 non-adults from Hazel Grove. Of these, 7 were females, 6 were males and 21 were of unknown sex (Table S2). The samples selected from both sites were well suited for isotopic analysis because of their good preservation. Age and sex for both sites were determined osteologically by Newman and Holst (2016) and Keefe and Holst (2017). The possible females (?F) and possible males (?M) were treated as females and males, respectively, in this dataset. A total of 111 faunal remains representing a range of species were sampled, 37 from Cross Street and 74 from Norton Priory (Table S3).
Methods
Bone collagen isotope analysis
Collagen extraction of bone followed the standard operating procedure for BioArCh at the University of York, based on the Longin (1971) method modified by Brown et al. (1988). Each bone sample was cleaned using powder abrasion, to remove contaminants of the outer layer of bone. Following this, bone samples (300–400 mg) were demineralised in 8 ml of 0.6 M hydrochloric acid (HCl) for 1–5 days. The demineralised samples were rinsed thrice using deionised water and then gelatinised using pH3 HCl on a heat block for 48 h at 80 °C. Subsequently, the soluble collagen solutions were Ezee™ filtered to remove unwanted particulate matter from the collagen solution, and then the supernatants were then ultrafiltered to isolate the high molecular weight > 30 kDa fraction. They were then frozen for a minimum of 12 h at −20 °C before being lyophilized in a freeze dryer for 48 h. Collagen yields (wt.%) were calculated to distinguish well-preserved from poorly preserved collagen using the formula [(bone mass (mg)/bone collagen mass(mg)) × 100]. Between 0.9 and 1.1 mg of the freeze-dried retentate were weighed out in duplicate into 4 × 3.2 mm tin capsules and combusted alongside international standards in an Elemental Analyser/Isotope Ratio Mass Spectrometer (EA/IRMS), a Sercon 20-22 mass spectrometer coupled with a Sercon GSL Sample Preparation System module at BioArCh, University of York.
All δ13C and δ15N ratios are expressed using the delta notation (δ) in parts per thousand (‰) relative to the international standards, VPDB for δ13C and atmospheric N2 (AIR) for δ15N, using the following equation:
where iE and jE denote the heavier and lighter isotopes, respectively (Roberts et al. 2018). Accuracy was determined by measurements of international standard reference materials within each analytical run. These were IAEA 600 ẟ13Craw = − 27.65 ± 0.09‰, ẟ13Ctrue = − 27.77 ± 0.043‰, ẟ15Nraw = 0.92 ± 0.21‰, ẟ15Ntrue = 1 ± 0.2‰; IAEA N2 ẟ15Nraw = 20.35 ± 0.13‰, ẟ15Ntrue = 20.3 ± 0.2‰; IA Cane, ẟ13Craw = − 11.77 ± 0.09‰; ẟ13Ctrue = − 11.64 ± 0.03‰.
We calculated the overall uncertainties on the measurements of each sample based on the method of Kragten (1994) by combining uncertainties in the values of the international reference materials and those determined from repeated measurements of samples and reference materials. These are expressed as one standard deviation. The maximum uncertainty for all samples across all runs was < 0.2‰ for both δ13C and δ15N values. In addition, a homogenised bovine bone extracted and analysed within the same batch as the samples produced the following average values: ẟ13C = −23.01 ± 0.13; δ15N = 6.21 ±0.44. This was comparable to the overall mean value from 50 separate extracts of this bone sample, which produced values of ẟ13C = −22.97 ± 0.19 and δ15N = 6.19 ± 0.30.
Bayesian stable isotope mixing models
For quantitative dietary estimation to aid in interpretation, the Bayesian stable isotope mixing model software FRUITS (Food Reconstruction Using Isotopically Transferred Signals) was employed in this study (Fernandes et al. 2014). This model estimates the relative contribution (%) of different food sources to the human diet. The dietary proxies used in these model estimations were δ13Ccollagen and δ15Ncollagen with the addition of published δ13Capatite values for Cross Street and Hazel Grove from Chidimuro et al. (2023). The uncertainty was set at 0.5‰. Four food sources were considered: C3 plants (δ13C = −24.0 ± 0.2‰; δ15N = 6.3 ± 0.8‰), C4 sugar and maize (δ13C = −10.4 ± 1.3‰; δ15N = 4.0 ± 0.6‰), terrestrial herbivores (sheep and cattle: δ13C = −22.1 ± 0.0‰; δ15N = 6.6 ± 0.2‰) and terrestrial omnivores (pigs and domestic fowl: δ13C = −21.3 ± 0.1‰; δ15N = 8.2 ± 0.3‰). For C3 plants, the average δ13C and δ15N values of barley from Iron Age Switzerland were utilised (Knipper et al. 2017). The δ15N values were corrected for the charring effect (+0.3‰) (Nitsch et al. 2015). The C4-based food values were obtained from the study of Nash et al. (2012) using modern market sugar and corn. To account for the atmospheric Suess effect in modern cane sugar and corn values, the δ13C values were adjusted by +2‰ using the atmosphere in 2010 (Hellevang and Aagaard 2015). The average δ13C and δ15N values of herbivores and omnivores were generated in this study from animals sampled from Cross Street and Norton Priory. The uncertainties associated with the food values are standard errors of the mean.
For each food source, the mean δ13C and δ15N values of protein and energy were estimated using fractionations reported by Fernandes (2016). The following fractionations between macronutrient isotope composition were utilised—Plants: Δ13Cprotein-bulk = −2‰, Δ13Ccarbohydrates-bulk = +0.5‰, Δ15Nprotein-bulk = 0‰; Terrestrial animals: Δ13Cprotein-collagen = −2‰, Δ13Clipids-collagen = −8‰, Δ15Nprotein-collagen = +2‰ (Fernandes 2016). Following Fernandes (2016), Δ13Capatite-diet was set at +10.1 ± 0.5‰, routed 100% from bulk δ13C diet; Δ15Ncollagen-diet was set at +5.5 ± 0.5‰, with 100 % contribution from protein; and Δ13Ccollagen-diet was set to +4.8 ± 0.5 ‰, with 74 ± 4% contribution from protein and 26 ± 4% from lipids and carbohydrates. Three food fractions were utilised: bulk, proteins and energy (lipids and carbohydrates). Prior information used was ([Protein]/([Protein] + [Energy])) > 0.05; ([Protein]/([Protein] + [Energy])) < 0.4.
ZooMS analysis
Since we could not separate the sheep and goat samples from Cross Street, they were distinguished on the basis of ZooMS (Zooarchaeology by Mass Spectrometry) following the biomolecular methods used in Buckley et al. (2009) and Buckley et al. (2010). The same collagen that was used to measure the stable isotopes in the present study was sequenced for ZooMS, and all samples ran in triplicate. A volume of 100 µL of 0.05 M ammonium bicarbonate solution was added to each collagen sample. Following this, samples were incubated at 65 °C for 1 h and then centrifuged at 14,000 RCF for 1 min. After centrifugation, 50 µL of the supernatant was transferred to a fresh Eppendorf tube, and 0.4 µg of sequencing grade trypsin was added. Samples were then incubated overnight at 37 °C, and enzymatic termination was performed by acidification of the solution with 1 µL of 5% v/v trifluoroacetic acid. Peptides were desalted via C18 ZipTips and then spotted onto a MALDI target plate for analysis. Replicates were averaged using the open-source software mMass (Strohalm et al. 2010), and the m/z values were examined for the presence of published peptide markers to differentiate between sheep and goat (Buckley et al. 2010). All 13 sheep/goat samples from this site were identified as sheep.
Statistical analysis
Statistical analysis was undertaken using R statistics, PAST, and SPSS Statistics version 26 to compare the diets of all remains in this study using an alpha level of 0.05 for all statistical tests (Hammer et al. 2001; IBM 2019; R Core Team 2020). Non-parametric statistics were used to compare diagenetic parameter values between the population groups and the variations within them because of the non-normal distribution of data as indicated by Kolmogorov-Smirnov and Shapiro-Wilk tests. The tests were executed using the non-parametric equivalent of a one-way analysis of variance (ANOVA) and the non-parametric equivalent of the independent T-test: Kruskal-Wallis test and Mann-Whitney U test, respectively.
Results
Tables S2 and S3 summarise the isotopic data for the human and faunal samples. Collagen preservation was assessed through collagen yields (%), elemental (%C, %N) data and atomic C:N ratios. The collagen yields from Cross Street and Hazel Grove samples were in the range of between 1.2 and 22.6% and 3.0 and 16.1%, respectively (Table S2). Good bone collagen preservation for faunal remains was indicated by collagen yields (faunal range 3.7 to 25.3%; See Table S3). A decrease in collagen yield is to be expected due to ultrafiltration (Jørkov et al. 2007); therefore, samples with a collagen yield of less than 1% were not excluded but subjected to further quality checks. For both human and faunal remains, elemental (%N, %C) data from all samples exceeded acceptable standards of at least 4.8% and 13% for nitrogen and carbon, respectively (Ambrose 1990, 447). The atomic C:N ratios from both the human and faunal remains fell within the acceptable range of between 3.1 and 3.5 (van Klinken 1999).
Animal data
The isotopic data for animals from both Cross Street and Norton Priory are similar, with data largely falling out between species with different feeding habits at both sites (Table 1).
The δ13C values for all animals bar one pig from Cross Street are within the range of C3 terrestrial diets (Fig. 2). Pig CSM 6217, however, possesses a δ13C value of −16.2‰ and a relatively low δ15N value of 6.4‰ indicative of a measurable C4 contribution to the diet. There is no statistical difference between δ13C values for cattle, pigs and sheep and δ15N values for cattle and pigs from both sites, although sheep do possess statistically different δ15N values between sites as a result of the higher overall δ15N values for Norton Priory when compared to Cross Street (Table 2).
Comparison with other post-medieval animal remains
The isotopic values for the animals from northwest England plot similarly to the same species published elsewhere from London and Durham, although omnivorous domestic fowl tend to show a variable δ15N values between sites (Fig. 3).
Animal mean δ13C and δ15N values ± 1σ (error bars) from Cross Street Manchester (CSM) and Norton Priory Chester (NP). There are only two domestic fowl from Cross Street; therefore, they have not been averaged. Dotted error bars derive from published post-medieval data from Queen’s Chapel Savoy and Prescot Street sites in London (Lon, Bleasdale et al. (2019)) and from Durham (Dur, Millard et al. (2015))
The sheep from Norton are, however, again notable for their high δ15N values. The δ13C values of the cattle, pigs and sheep from this study generally fall within the range of values reported for post-medieval Queen’s Chapel Savoy and Prescot Street sites in London as well as in Durham (Millard et al. 2015; Bleasdale et al. 2019) (Fig. 3). There are no statistically significant differences between the cattle and pigs from the Cross Street, Norton Priory and London sites (Table S4). Comparisons between sheep from this study and the London and Durham sheep showed no significant differences in the δ13C values among the four groups, but there are statistically significant differences between the δ15N values. Post hoc analysis reveals that, as expected, the Norton Priory sheep are statistically different to those from London and Durham (Table S5).
Human data
The results of stable isotope analysis for all the human individuals are presented in Table S2 and plotted alongside the animal data in Fig. 4.
The δ13C values range from − 18.0 to − 20.7‰ (mean − 19.8 ± 0.5‰) for the Cross Street population and from − 19.2 to − 20.6‰ (mean − 20.1 ± 0.4‰) for the Hazel Grove population. The δ15N values range from 9.5 to 15.1‰ (mean 11.7 ± 1.1‰) and 9.4 to 13.9‰ (mean 11.1 ± 1.1‰) for the Cross Street and Hazel Grove populations, respectively. It is immediately apparent that there is a site-based difference, with the Cross Street population plotting with much higher δ13C and δ15N values than those from Hazel Grove (Fig. 4). This is further explored by examining adult and non-adult diets in turn below.
Adult diet
Adults from Cross Street (n = 54) have a mean δ13C and δ15N values (1σ) of − 18.1 ± 0.6‰ and 11.6 ± 0.9‰, respectively. In contrast, adults at Hazel Grove (n = 19) have lower values for both δ13C and δ15N and smaller standard deviations at − 20.2 ± 0.3‰ (1σ) and 10.8 ± 0.6‰ (1σ), respectively. The overall range in adult δ13C and δ15N values is therefore smaller at Hazel Grove in comparison to Cross Street, standing at 1.4‰ vs. 2.7‰ for δ13C and 2.3‰ vs. 3.5‰ for δ15N. Four individuals at Cross Street are particularly distinctive for high δ13C values > − 19‰, and in terms of δ15N, only individuals from Cross Street plot above ~ 12‰. The differences between the two adult populations are statistically significant for both δ13C and δ15N (Mann-Whitney U test U = 235.5, p = < 0.001 and U = 230.5, p < 0.001, respectively).
When compared with the animal data (Fig. 4), the δ13C and δ15N values in the adults suggest that dietary protein is primarily derived from animal sources. The Δ13Chuman-herbivore for Hazel Grove is 1.8‰; however, at Cross Street, the Δ13Chuman-herbivore of 2.3‰ is just above the upper limit of 0–2‰ (Lee-Thorp et al. 1989; Bocherens and Drucker 2003). The offsets in δ13C values between adult humans and omnivores (Δ13Chuman-omnivores) for both the Cross Street and Hazel Grove populations are 1.4‰ and 0.9‰, respectively. Regarding human-fauna offsets in nitrogen isotope values, the offsets between the Cross Street and the Hazel Grove populations and herbivores (5.0‰ and 4.0‰, respectively) and Cross Street omnivores (3.5‰) fall within the expected trophic level enrichment of 3–5‰ (Schoeninger 1985; Bocherens and Drucker 2003), but those from Hazel Grove fall below the lower limit of 3‰ (δ15NHazel Grove-omnivores = 2.5‰) indicating a similar trophic level.
Sex differences in diet are explored for both populations in Fig. 5.
Bone collagen δ13C and δ15N values for a females (n = 27 including 1 sexed adolescent), males (n = 25) and unknown sex (n = 3) from Cross Street; b Intra-population diversity (sex and possible African/mixed Ancestry (AA)) bone collagen δ13C and δ15N values for females (n = 7), males (n = 6) and unknown sex (n = 6) from Hazel Grove
At Cross Street, males (n = 25) show a mean δ13C value of − 19.7 ± 0.6‰ (1σ); and mean δ15N value of 11.6 ± 1.0‰ (1σ), with females (n = 27) having similar mean values for δ13C = − 19.8 ± 0.6‰ and δ15N = 11.7 ± 0.8‰. At Hazel Grove, males (n = 6) have a mean δ13C value of − 20.4 ± 0.1‰; and δ15N value of 10.9 ± 0.8‰ and females (n = 7), similar mean values for δ13C = − 20.1 ± 0.4‰ and δ15N = 10.8 ± 0.5‰. The lack of differences between males and females at Cross Street is borne out in statistical tests (Mann–Whitney U test, δ13C: U = 367, p = 0.589; δ15N: U = 347, p = 0.862). There is also no statistical difference in δ13C values between sexes at Hazel Grove (δ13C: U = 9.5, p = 0.110), but there is a statistical difference in δ15N (δ15N: U = 253.5, p < 0.001), with men having slightly higher δ15N values than women.
A further comparison was possible for Hazel Grove, where four individuals had been identified as being of possible African or mixed ancestry (SK 1, SK 10, SK 14 and SK 21), indicated in Fig. 5b. These individuals on the whole plot similarly to the rest of the adult population from the site (Fig. 5b). Although one individual (SK 14) has a slightly higher δ13C value, they are within two standard deviations of the mean and cannot be identified as a statistical outlier.
Each adult population was further broken down into osteological adult age categories to explore dietary dynamics with age, plotted in Fig. 6, with descriptive statistics presented in Table 3. There are no differences in diet between the adult age categories at both sites (Fig. 6). This is also supported statistically in the Cross Street adult population adult age groups where there are sufficient number of individuals to examine (18 + years, 26–35 years, 36–45 years and 46 + years, Kruskal–Wallis H test, δ13C: X2(3) = 6.215, p = 0.102 and δ15N: X2(3) = 3.981, p = 0.264).
Non-adult diet
The δ13C and δ15N values for the non-adults are summarised in Table 4 and plotted individually by their average age in comparison with the adult mean at both sites in Fig. 7.
Many of the non-adults from both sites fall within 1σ of their respective adult mean, with some variation (Fig. 7). The peak δ13C values are observed in two individuals aged between 0 and 2 years and two aged 4 and 15 years old from Cross Street and those aged between 0 and 3 years at Hazel Grove (Fig. 7a). At the younger ages, this could conceivably represent the influence of breastfeeding. Nitrogen isotope values are more variable. At Cross Street, δ15N values higher than the adult mean (+/−1σ) are observed in five Cross Street non-adults under the age of 4 years old as well as an adolescent. There are additionally five non-adults between the age of 4 and 12 years old that exhibit lower δ15N values compared with the adult population (Fig. 7b). At Hazel Grove, the highest δ15N values are observed in nine Hazel Grove non-adults clustered around 0–4 years old before δ15N values then decrease. There is only one individual that plots firmly within one standard deviation of the adult mean. This likely reflects dietary and physiological stress in these early years of life.
Omitting individuals under the age of 2 who have the added complication of breastfeeding, there are sufficient non-adults within each osteological age category at Cross Street (> 2 years to 6 years, 7–12 years and 13–17 years) (Keefe and Holst 2017), to perform a statistical comparison by age. No statistically significant differences were found for either δ13C or δ15N (Kruskal-Wallis H test, δ13C: X2(2) = 2.508, p = 0.285 and δ15N: X2(2) = 0.281, p = 0.869). Comparison between all non-adults combined (> 2 years old, n = 31) and adults (n = 54) for both δ13C and δ15N in this population also shows no statistical difference (Mann–Whitney U test U = 680.5, p = 0.299 and U = 744, p = 0.662, respectively).
This dietary depiction is further supported by the Bayesian model FRUITS confirming the dietary differences between Cross Street and Hazel Grove (Fig. 8).
FRUITS model showing the contribution of C3 plants, herbivores, C4 cane sugar and maize and omnivores in post-medieval Cross Street (CSM) and Hazel Grove (HGM) populations. Boxes represent a 68% credible interval (corresponding to the 16th and 84th percentiles), while the whiskers represent a 95% credible interval (corresponding to the 2.5th and 97.5th percentiles). The horizontal dashed line represents the estimated mean, while the horizontal discontinuous line represents the estimated median (50th percentile)
It can be observed that C3 plant resources provided a greater contribution of an average of about 55% and 64% at Cross Street and Hazel Grove populations, respectively. This was followed by herbivores which were 29% and 24%, respectively. The omnivores and C4 cane sugar and maize contribute the least. The omnivores contribute about 8% and 5%, respectively, while the C4-based foods contribute 8% and 7%, respectively. It must be noted that the terrestrial animal and C4-based food contribution are higher in Cross Street individuals compared to the Hazel Grove population.
Comparison with other post-medieval populations: human data
Isotope data from humans from the Manchester populations is compared with data from seven contemporaneous cemeteries in England in Fig. 9, including London (Bleasdale et al. 2019; Beaumont et al. 2013b; Nitsch et al. 2010; Trickett 2006), Chichester (Dhaliwal et al. 2020), Coventry (Trickett 2006) and Birmingham (Richards 2006).
A biplot of δ13C and δ15N values, showing mean and 1σ for individuals from Cross Street (CSM) and Hazel Grove (HGM) Manchester and eight contemporary English sites: St. Michael’s Litten cemetery, Chichester, separated into three social classes (ESC) (Dhaliwal et al. 2020); St. Barnabas (SBK) and Queen Chapel Savoy (QCS) (Bleasdale et al. 2019); Lukin Street (LS) (Beaumont et al. 2013b); Spitalfields, London (SL) (Nitsch et al. 2010); St. Luke’s Chelsea (SLC) and Holy Trinity, Coventry (HTC) (Trickett 2006) and Churchyard of St. Martin’s-in-the-Bull Ring, Birmingham (SMB) (Richards 2006)
All populations except the lower-status Chichester individuals (buried in shrouds) display higher δ13C and δ15N values when compared to both Cross Street and Hazel Grove, with the latter having the lowest mean δ13C and δ15N values of all comparative populations (Fig. 9).
Site data was explored statistically for published populations with similar socioeconomic status to those at Hazel Grove and Cross Street, with middle/high and low socioeconomic class populations being assessed separately. Queen’s Chapel Savoy was excluded as a mixed-status population including hospital patients, parishioners, criminals and military personnel (Bleasdale et al. 2019). There were statistically significant differences between Cross Street and the comparative middle/high socioeconomic class populations (Kruskal-Wallis H test, δ13C: X2(6) = 124.1, p = 0.000 and δ15N: X2(6) = 174.8, p = 0.000) and between Hazel Grove and the low socioeconomic class populations (Kruskal-Wallis H test, δ13C: X2(3) = 56.30, p = 0.000 and δ15N: X2(3) = 62.99, p = 0.000). Post hoc pairwise tests adjusted using Holm’s Sequential Bonferroni correction produced during the tests are presented in Table 5.
Discussion
Animal management
The herbivore (cattle and sheep) carbon and nitrogen stable isotope data from this study indicate that they consumed a diet mainly consisting of C3 terrestrial resources, conforming to the expectations that livestock fed on C3 forage and fodder during the post-medieval period. However, the omnivores (pigs and domestic fowl) have slightly higher mean δ13C and δ15N values than herbivores, suggesting diets enriched in both 13C and 15N. The differences between δ15N values for herbivores and omnivores are indicative of omnivores consuming high trophic level protein, likely in the form of food scraps discarded by humans (Müldner and Richards 2007; Hammond and O’Connor 2013). One pig in particular (CSM 6217) exhibited a very high δ13C value (− 16.2‰) and low δ15N (6.4‰), implying that it might have been fed on C4 plants, most likely to be maize that was used for fodder in industrial Britain (Brassley 2000; Martin 2012). This is the first clear indication of a C4-fed pig in post-medieval England, indicating the value of an isotopic approach in revealing direct evidence of this foddering regime. It is also possible, however, that the pig might have originated from a different country where it was fed a C4 diet. There is evidence that pigs were imported from the Americas, Australia, Africa and Canada during the eighteenth century (Capie and Perren 1980; Plumb 2010). Similar values have been reported by Guiry et al. (2015) for one of the William Salthouse salted pigs (LTU 26) from Australia bound for Canada; therefore, it is possible that CSM 6217 could have been an imported pig.
Compared to the livestock from London and sheep from Durham, differences are only observed between the δ15N values in sheep from Norton Priory that exhibit a wide range of δ15N values (+4.6‰ to 10.5‰). Norton Priory is located in the Mersey Estuary, which contains areas of coastal saltmarsh that were likely more extensive in the past than today (Langston et al. 2006). Plant species found in saltmarsh areas exhibit enrichment in 15N (Britton et al. 2008), and it is probable that the elevated δ15N values for sheep from this site, in particular, reflect the use of local saltmarsh for grazing, a practice that continues in the region (Langston et al. 2006). The wide range in isotopic values of the sheep at Norton Priory, however, also points to them deriving from a geographically wide range during this period (Drummond and Wilbraham 1939). The technological developments of steamships and railways in the nineteenth century eased the transportation of these animals from longer distances. Historical documents, for example, indicate that in order to satisfy the demand for sheep in Cheshire during this period, livestock was brought from the Welsh borders (Marshall 1818; Scola 1992) or London (Drummond and Wilbraham 1939; Beaumont et al. 2013b; Bleasdale et al. 2019).
Socioeconomic influence on human diets in Manchester
The δ13C and δ15N values of individuals from both cemeteries in post-medieval Greater Manchester indicate that their diet consisted of C3 terrestrial plants as well as meat and/or other animal products from animals foddered on C3 resources. However, diet and social life were inextricably linked in post-medieval England (Bleasdale et al. 2019), and greater inter-population diversity in the δ13C and δ15N values between the Cross Street and Hazel Grove populations is likely to be consistent with their diverse social status. High δ13C and δ15N values for some of the Cross Street adults may be due to the consumption of more 13C enriched foods, marine sources or high trophic level animal protein (Schoeninger and DeNiro 1984), which were unavailable or consumed in lower quantities by the mostly lower-class Hazel Grove population (Scola 1992). As expected from what we know from historical accounts, the more affluent population from Cross Street seems to have had greater access to high trophic level animal protein, e.g. meat. Nonetheless, the adult human-animal offsets in δ15N values at Cross Street were not very high for both herbivores and omnivores (5.0 ‰ and 3.5 ‰, respectively). These values fall within the expected trophic level enrichment of 3–5‰ (Schoeninger 1985; Bocherens and Drucker 2003), which suggests that marine resources were probably not a significant component of the diet, but the high δ15N values for sheep may be driving up the mean herbivore δ15N value and lessening the offset between humans and herbivores. In this light, marine resources, certainly those of lower trophic levels, cannot be disregarded, particularly as historic sources record fish being supplied to the city (Aikin 1785; Chaloner 1959). The δ13C offsets of Cross Street adult humans against herbivores (2.3‰), on the other hand, are just above the upper limit of the trophic level shift of 0–2‰ (Lee-Thorp et al. 1989; Bocherens and Drucker 2003), whereas that of Hazel Grove is lower and within the trophic level shift of 0–2‰, suggesting consumption of more 13C enriched foods or animals foddered on C4 resources for Cross Street individuals (Schoeninger and DeNiro 1984). Although maize, a C4 plant, was available in post-medieval England, historical sources indicate that it was considered to be animal fodder or a famine relief food for the Irish poor (Holland 1919; Ó’Gráda 1989) and with the majority of the Cross Street population being middle class, direct consumption of C4 foods was most likely to be sugar from sugarcane (Walvin 2017). Notably, however, recent analysis of dental calculus micro-debris from 36 individuals from the Cross Street population revealed the presence of maize in two skeletons, one of which is studied here (SK1.12; (MacKenzie et al. 2021), suggesting direct consumption. MacKenzie et al. (2021) suggest that either these two individuals were lower class, since not all Cross Street individuals were necessarily middle or upper class or they lived on poor relief for part of their life but become wealthier in later life. Additionally, the authors suggested it could be related to cotton processing, since maize starch was utilised in the treatment of cotton; therefore, it was breathed in rather than consumed. Sugarcane and maize, however, are plants with little to no protein content and are unlikely to affect bone collagen isotope values as 75% of the carbon in bone collagen is derived from dietary protein, whereas only about 25% is derived from fats and carbohydrates (Fernandes et al. 2012). Further analysis of bone apatite to examine the whole diet is required to fully explore the potential dietary input of C4 sugar/maize (Ambrose et al. 1997).
The period of breastfeeding and weaning for these populations is difficult to interpret using bone collagen data. The patterning for δ13C values for non-adults from both populations indicates that the highest values tend to occur between the ages of 0 and 2 years and thereafter they generally decline. These high δ13C values are not correlated with lower δ15N values as seen in other studies that postulate that an increase in δ13C values may represent the input of sugar as a weaning food in the infant diet (Nitsch et al. 2011; Waters-Rist et al. 2022). Carbon isotope values of bone collagen are considered to be more robust for assessing the introduction of weaning food, potentially being less affected by physiological stress (Beaumont et al. 2018), so this patterning could indicate weaning initiated at around 2 years old. The δ15N values for infants from both populations, however, are not straightforward, with individuals from both sites having elevated δ15N values up to the age of around 4 years. It is unlikely that breastfeeding continued to this age in this period (Britton et al. 2018), and instead, these values likely reflect severe physiological and metabolic stress in both populations, which can manifest in high δ15N values (Katzenberg and Lovell 1999; Fuller et al. 2005; Mekota et al. 2006). A recent study by Newman and Gowland (2017) demonstrated that during the eighteenth and nineteenth century, all children were susceptible to health and dietary stress regardless of status. Infant diet during this period was subject to variation dependent on individual needs or preferences. Infants from the middle or upper classes may have been weaned early onto poor diets because breastfeeding was considered inconvenient or unfashionable, while early weaning by low classes could be associated with mothers working outside the home, for example urban factory workers (Waters-Rist et al. 2022). Instead, infants were often given foods with little nutritious value such as pap (a mixture of flour or breadcrumbs cooked in milk or water), panada (a bread broth or milk flavoured with sugar, eggs or spices) or cow’s milk (Drummond and Wilbraham 1939; Nitsch et al. 2011; Britton et al. 2018) with the poorest in society not being able to afford animal milk. Infant mortality was much higher at Hazel Grove than Cross Street, with almost half (42.1%) of the non-adult population consisting of infants and the highest incidence of rickets and scurvy in the population. The incidence of these conditions was three times more prevalent at Hazel Grove compared with Cross Street overall. High infant mortality in this area of Greater Manchester at the time is corroborated by historical records, stating that 45% of all deaths in Stockport in 1850 were in those under 5 years of age (Registrar-General 1854). To explore the patterns in infant diets seen here further, incremental sectioning of tooth dentine could potentially offer a more reliable assessment of periods of breastfeeding and weaning in these populations (Beaumont et al. 2018).
An assessment of access to food resources within the two populations themselves yields interesting differences between the two sites. The δ13C and δ15N results indicate that access to food was similar across all age groups at Cross Street. Comparison between the older non-adults from both sites shows those from Cross Street were consuming more animal/high trophic level protein than the Hazel Grove non-adults, in line with the results obtained for the adults. This is in keeping with historical evidence indicating that middle-class children generally consumed the same food as adults (Turner 1995). On the other hand, the non-adults from Hazel Grove consumed foods less enriched in 15N when compared to adults, suggesting that they were provided with a diet lower in animal protein. Similar patterns to Hazel Grove in isotopic values were seen between non-adults and adults at post-medieval Spitalfields in London (Nitsch et al. 2011). The provisioning of children with poorer diets in comparison to adults is known to have occurred in lower-class populations in the industrial past because there was not sufficient food for everyone and those working were prioritised (Rowntree 1901). When combining isotopic data with osteological evidence, which shows limited metabolic disease at Cross Street and a high incidence (scurvy and rickets, particularly in infants) and childhood stress indicators for Hazel Grove, it suggests that it is the inadequate diet that children in the Hazel Grove population experienced, which caused metabolic and physiological stress. Additionally, the significant social and environmental pressures experienced in early life appear to have been a major contributing factor to high infant mortality at Hazel Grove.
There were no differences regarding access to food between the males and females in the Cross Street population. An account by Davidoff and Hall (2018) of men and women of the English middle class between 1780 and 1850 suggested that during this time, middle-class gender roles were flexible, with some women working in businesses and other occupations as much as men. Therefore, one would not necessarily expect dietary differences between the sexes. However, there is a statistical difference in the δ15N between men and women at Hazel Grove suggesting distinct protein intake between the two groups, albeit based on very few individuals. This tentatively aligns with information from various sources indicating that a greater quantity, or better quality food, such as meat, was reserved for working men who often undertook physically demanding jobs to feed the family (Broomfield 2007; Clayton and Rowbotham 2008, 2009), and this was reflected in males’ higher nitrogen values at Hazel Grove compared to that of women and children.
Comparison with contemporary sites in England: human data
Individuals from St. Luke’s, St. Barnabas, Spitalfields, Birmingham and higher-class Chichester (buried in coffins and tombs) were classified as being from middle/higher socioeconomic classes, whereas those from Lukin Street, Coventry and Chichester (shroud burials) were classified as being from low economic classes based on cemetery records (Richards 2006; Trickett 2006; Nitsch et al. 2010; Beaumont et al. 2013b; Bleasdale et al. 2019; Dhaliwal et al. 2020). The middle/higher socioeconomic class populations from St. Luke’s, St. Barnabas, Spitalfields, Birmingham and Chichester all have higher δ13C and δ15N values than the lower-class populations (Fig. 9). Despite being lower class, the populations from Lukin Street and Coventry both display higher δ13C and δ15N values than the middle-class Cross Street population. However, the lower-class Chichester individuals have the lowest δ15N values among all sites.
There is no statistical difference between Cross Street and both the middle- and higher-class individuals from Chichester (Table 5) suggesting similarities in their diet along similar social classes in two different regions of England. This does not, however, extend to the rest of the comparative populations which demonstrate significant differences in isotope values despite similarities in socioeconomic status. The Cross Street and St. Martin’s Birmingham populations have similar δ15N isotope values but significantly different δ13C isotope values, whereas the Birmingham population has higher δ13C isotope values. It is possible that, because the city of Birmingham was wealthier than Manchester during the nineteenth century (Wise and O’Thorpe 1950; Wise 1951), St. Martin’s individuals had more access to C4-fed animal protein, such as the pig seen in this study, compared to the Cross Street individuals, which may account for the differences in the δ13C values between the two populations despite their similar status. However, although this difference is statistically significant, it is only 0.8‰, which is too small to imply a pronounced difference in diet. All of the London populations have higher mean δ13C and δ15N values, and since isotopic animal data is similar between London and the northwest, rather than a baseline shift in isotopic values, we are seeing a dietary difference between these populations. This suggests that London’s access to 13C-enriched foods such as cane sugar or maize (Beaumont 2013; Beaumont et al. 2013b; Bleasdale et al. 2019) and/or high trophic level or marine protein was greater than that of other contemporary English cities (Spencer 2000; Picard 2006; Trow-Smith 2013; Metcalfe 2015). Easier access to meat and fish for the London individuals due to improvements in transportation and interventions from the government that lowered fish prices and increased the number of livestock brought to the city might explain the higher isotope values here (Drummond and Wilbraham 1939; Stead 1985; Burnett 2004).
Conclusions
Isotopic analysis of two populations from post-medieval Greater Manchester has provided a detailed insight into diet and social status in an urban context in Northern England. The large animal dataset dramatically expands the extant post-medieval animal isotopic information and revealed differences between species that likely reflects the wide geographic area that the city drew livestock from, representing a range of environments and management practices, including a pig fed on C4 plants and probable grazing of sheep on the saltmarshes of the Mersey Estuary. The diet of the Manchester human populations was predominantly based on plants and animal protein from C3 terrestrial environments, common for England during this time. Elevated δ13C isotope values from middle-class Cross Street could suggest that these individuals had minor contributions of C4 resources (e.g. cane sugar) or perhaps marine foods, as observed in some of the individuals from this site when bone carbonate analysis was applied (Chidimuro et al. 2023). Further analysis of single amino acid analysis of collagen (Chikaraishi et al. 2010) could provide greater clarity.
Notably, diet was differentiated along socioeconomic lines, where those representing the lower social classes at Hazel Grove had less access to high trophic level protein (meat/milk) than those at middle-class Cross Street. While the Cross Street population enjoyed a similar diet across all demographic groups, the data suggests that male adults at Hazel Grove were provided with a better quality diet compared to females and children, probably to support their role as breadwinners. Such dietary distinction likely caused the greater non-adult mortality rates and stress indicators in the Hazel Grove non-adults compared to those from Cross Street. Comparative analysis from London sites and other parts of England differentiated the London diet, suggesting greater access to high trophic level animal protein and aquatic/marine resources in London, even for those of lower social status compared to the Manchester populations. Expanding these analyses to additional populations, especially from the north of England, would assist in developing a detailed bioarchaeological picture of access and diversity in diet and the resulting stark differentiation in lifeway quality that characterises this tumultuous period of urbanisation in seventeenth to nineteenth century England.
Data availability
The data that supports the findings of this study are available in the supplementary material of this article.
Code availability
Not applicable.
References
Aikin J (1785) Description of the country from thirty to forty miles round Manchester. Johnson, London
Ambrose SH (1990) Preparation and characterization of bone and tooth collagen for isotopic analysis. J Archaeol Sci 17:431–451. https://doi.org/10.1016/0305-4403(90)90007-R
Ambrose SH, Butler BM, Hanson DB, Hunter-Anderson RL, Krueger HW (1997) Stable isotopic analysis of human diet in the Marianas Archipelago, Western Pacific. Am J Phys Anthropol 104:343–361. https://doi.org/10.1080/03122417.2001.11681705
Baker ST (1884) Memorials of a dissenting chapel, a sketch of the rise of nonconformity in Manchester, and of the erection of the Chapel in Cross Street. Simpkin, Marshall & Co, London
Banken E (2018) Assessing osteological indicators of ancestry through ancient genomic analyses: 10 British skeletons of putative African ancestry. BSc Dissertation, University of York
Barker TC, Oddy DJ, Yudkin J (1970) The dietary surveys of Dr. Edward Smith 1862–3: a new assessment. The dietary surveys of Dr. Edward Smith 1862–3. A new assessment. Univ. of London, London
Beaumont J, Gledhill A, Lee-Thorp J, Montgomery J (2013a) Childhood diet: a closer examination of the evidence from dental tissues using stable isotope analysis of incremental human dentine. Archaeometry 55:277–295. https://doi.org/10.1111/j.1475-4754.2012.00682.x
Beaumont J, Geber J, Powers N, Wilson AS, Lee-Thorp J, Montgomery J et al (2013b) Victims and survivors: stable isotopes used to identify migrants from the Great Irish Famine to 19th century London. Am J Phys Anthropol 150:87–98. https://doi.org/10.1002/ajpa.22179
Beaumont J, Atkins E, Buckberry J, Haydock H, Horne P, Howcroft R, Mackenzie K, Montgomery J (2018) Comparing apples and oranges: why infant bone collagen may not reflect dietary intake in the same way as dentine collagen. Am J Phys Anthropol 167:524–540. https://doi.org/10.1002/ajpa.23682
Beaumont J (2013) An isotopic and historical study of diet and migration during the Great Irish Potato Famine (1845–1852): high-resolution carbon and nitrogen isotope profiling of teeth to investigate migration and short-term dietary change at the union workhouse, Kilkenny and Lukin Street, London. PhD Thesis, University of Bradford
Blackman J (1975) The cattle trade and agrarian change on the eve of the Railway Age. Agric Hist Rev 23:48–62
Bleasdale M, Ponce P, Radini A, Wilson AS, Doherty S, Daley P et al (2019) Multidisciplinary investigations of the diets of two post-medieval populations from London using stable isotopes and microdebris analysis. Archaeol Anthropol Sci 11:6161–6181. https://doi.org/10.1007/s12520-019-00910-8
Bocherens H, Drucker D (2003) Trophic level isotopic enrichment of carbon and nitrogen in bone collagen: case studies from recent and ancient terrestrial ecosystems. Int J Osteoarchaeol 13:46–53. https://doi.org/10.1002/oa.662
Booth A (1977) Food riots in the North-West of England 1790–1801. Past Present 77:84–107
Brassley P (2000) Output and technical change in twentieth-century British agriculture. Agric Hist Rev 48:60–84
Britton K, Müldner G, Bell M (2008) Stable isotope evidence for saltmarsh grazing in the Bronze Age Severn Estuary, UK: implications for palaeodietary analysis at coastal sites. J Archaeol Sci 35:2111–2118. https://doi.org/10.1016/j.jas.2008.01.012
Britton K, McManus-Fry E, Cameron A, Duffy P, Masson-MacLean E, Czere O et al (2018) Isotopes and new norms: investigating the emergence of early modern U.K. breastfeeding practices at St. Nicholas Kirk Aberdeen. Int J Osteoarchaeol 28:510–522. https://doi.org/10.1002/oa.2678
Broomfield A (2007) Food and cooking in Victorian England: a history. Greenwood Publishing Group, London
Brown F, Howard-Davis C (2008) Norton Priory: Monastery to Museum: Excavations 1970–87. Oxford Archaeology North, Oxford
Brown TA, Nelson DE, Vogel JS, Southon JR (1988) Improved collagen extraction by modified Longin method. Radiocarbon 30:171–177
Brown C, Alexander M (2016) Hair as a window on diet and health in post-medieval London: an isotopic analysis. IA. https://doi.org/10.11141/ia.42.6.12
Buckley M, Collins M, Thomas-oates J, Wilson JC (2009) Species identification by analysis of bone collagen using matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry. Rapid Commun Mass Spectrom 23:3843–3854. https://doi.org/10.1002/rcm.4316
Buckley M, Kansa SW, Howard S, Campbell S, Thomas-Oates J, Collins M (2010) Distinguishing between archaeological sheep and goat bones using a single collagen peptide. J Archaeol Sci 37:13–20. https://doi.org/10.1016/j.jas.2009.08.020
Burnett J (2004) England eats out: a social history of eating out in England from 1830 to the present. Routledge, USA
Burnett J (2005) Plenty and want: a social history of food in England from 1815 to the present day. Routledge, USA
Byers SN (2010) Introduction to Forensic Anthropology, International. Pearson, Boston
Capie F, Perren R (1980) The British market for meat 1850–1914. Agr Hist Soc 54:502–515
CFA Archaeology Ltd (2017a) Cross Street Chapel, Manchester: archaeological excavations and exhumations. Assessment Report No. Y220/16, Volume 2. CFA Archaeology Ltd, Leeds
CFA Archaeology Ltd (2017b) Cross Street Chapel, Manchester: archaeological excavations and exhumations. Assessment Report No. Y220/16, Volume 1. CFA Archaeology Ltd, Leeds
Chaloner W (1959) Manchester in the latter half of the eighteenth century. Bull John Rylands Libr 42:40–60
Cherryson A, Crossland Z, Tarlow S (2012) A fine and private place: the archaeology of death and burial in post-medieval Britain and Ireland. University of Leicester, Leicester
Chidimuro B, Doherty S, Finch J, Ponce P, Eggington J, Delaney S et al (2023) North and South: exploring isotopic analysis of bone carbonates and collagen to understand post-medieval diets in London and northern England. Am J Biol Anthropol 182:126–142. https://doi.org/10.1002/ajpa.24818
Chikaraishi Y, Ogawa NO, Ohkouchi N (2010) Further evaluation of the trophic level estimation based on nitrogen isotopic composition of amino acids. In: Ohkouchi N, Tayasu I, Koba K (eds) Earth, life, and isotopes. Kyoto University Press, Kyoto, pp 37–51
Chisholm BS, Nelson DE, Schwarcz HP (1982) Stable-carbon isotope ratios as a measure of marine versus terrestrial protein in ancient diets. Science 216:1131–1132. https://doi.org/10.1126/science.216.4550.1131
Clayton P, Rowbotham J (2008) An unsuitable and degraded diet? Part one: public health lessons from the mid-Victorian working class diet. J R Soc Med 101:282–289. https://doi.org/10.1258/jrsm.2008.080112
Clayton P, Rowbotham J (2009) How the mid-Victorians worked, ate and died. Int J Environ Res Public Health 6:1235–1253. https://doi.org/10.3390/ijerph6031235
Cox G, Sealy J (1997) Investigating identity and life histories: isotopic analysis and historical documentation of slave skeletons found on the Cape Town Foreshore, South Africa. Int J Hist Archaeol 1:207–224. https://doi.org/10.1023/A:1027349115474
Davidoff L, Hall C (2018) Family fortunes: men and women of the English middle class 1780–1850. Routledge
Dhaliwal K, Rando C, Reade H, Jourdan A, Stevens RE (2020) Socioeconomic differences in diet: an isotopic examination of post-medieval Chichester, West Sussex. Am J Phys Anthropol 171:584–597. https://doi.org/10.1002/ajpa.23984
Douglas I, Hodgson R, Lawson N (2002) Industry, environment and health through 200 years in Manchester. Ecol Econ 41:235–255. https://doi.org/10.1016/S0921-8009(02)00029-0
Drummond J, Wilbraham A (1939) The Englishman’s food: a history of five centuries of English diet. Jonathan Cape Ltd, London
Engels F (1952) The condition of the working class in England in 1844: with a preface written in 1892; Reprinted 1952 with the Dedication Written by Engels in English in 1845. Allen & Unwin, London
Errington PA (2001) The history of Hazel Grove or should it be Bullock Smithy? [Online]. Available at: http://www.hazel-grove.com/history.html#short%20history. Accessed 4 Feb 2019
Fernandes R (2016) A simple(R) model to predict the source of dietary carbon in individual consumers. Archaeometry 58:500–512. https://doi.org/10.1111/arcm.12193
Fernandes R, Nadeau M-J, Grootes PM (2012) Macronutrient-based model for dietary carbon routing in bone collagen and bioapatite. Archaeol Anthropol Sci 4:291–301
Fernandes R, Millard AR, Brabec M, Nadeau MJ, Grootes PM (2014) Food reconstruction using isotopic transferred signals (FRUITS): a Bayesian model for diet reconstruction. PLoS ONE 9(2):e87436. https://doi.org/10.1371/journal.pone.0087436
Fuller BT, Fuller JL, Sage NE, Harris DA, O’Connell TC, Hedges REM (2004) Nitrogen balance and?15N: why you’re not what you eat during pregnancy. Rapid Commun Mass Spectrom 18:2889–2896. https://doi.org/10.1002/rcm.1708
Fuller BT, Fuller JL, Sage NE, Harris DA, O’Connell TC, Hedges RE (2005) Nitrogen balance and delta 15N: why you’re not what you eat during nutritional stress. Rapid Commun Mass Spectrom 19:2497–2506. https://doi.org/10.1002/rcm.2090
Glinert E (2009) The Manchester Compendium: A Street-by-street history of England’s Greatest Industrial City. Penguin, UK
Guiry EJ, Staniforth M, Nehlich O, Grimes V, Smith C, Harpley B, Noël S, Richards M (2015) Tracing historical animal husbandry, meat trade, and food provisioning: a multi-isotopic approach to the analysis of shipwreck faunal remains from the William Salthouse, Port Phillip, Australia. J Archaeol Sci Rep 1:21–28. https://doi.org/10.1016/j.jasrep.2014.10.001
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electronica 4:9
Hammond C, O’Connor T (2013) Pig diet in medieval York: carbon and nitrogen stable isotopes. Archaeol Anthropol Sci 5:123–127. https://doi.org/10.1007/s12520-013-0123-x
Hedges REM, Clement JG, Thomas CDL, O’Connell TC (2007) Collagen turnover in the adult femoral mid-shaft: modeled from anthropogenic radiocarbon tracer measurements. Am J Phys Anthropol 133:808–816. https://doi.org/10.1002/ajpa.20598
Hellevang H, Aagaard P (2015) Constraints on natural global atmospheric CO2 fluxes from 1860 to 2010 using a simplified explicit forward model. Sci Rep 5:17352
Hobson KA, Alisauskas RT, Clark RG (1993) Stable-nitrogen isotope enrichment in avian tissues due to fasting and nutritional stress: implications for isotopic analyses of diet. Condor 95:388–394
Hoefs J (2015) Stable isotope geochemistry, 7th edn. Springer International Publishing, Switzerland
Holland JH (1919) Food and fodder plants. Bull Misc Inform Kew 1919:1–84
Holt J (1794) General view of the agriculture of the County of Lancaster: with observations on the means of its improvement. J. Nichols, London
IBM (2019) IBM SPSS Statistics for Windows, Version 26.0. IBM Corporation, Armonk, NY, USA
Jørkov MLS, Heinemeier J, Lynnerup N (2007) Evaluating bone collagen extraction methods for stable isotope analysis in dietary studies. J Archaeol Sci 34:1824–1829. https://doi.org/10.1016/j.jas.2006.12.020
Katzenberg MA, Lovell NC (1999) Stable isotope variation in pathological bone. Int J Osteoarchaeol 9:316–324. https://doi.org/10.1002/(SICI)1099-1212(199909/10)9:5%3c316::AID-OA500%3e3.0.CO;2-D
Keefe K, Holst M (2017) Osteological analysis Cross Street Unitarian Chapel Manchester. Report No 0817 CFA Archaeology Ltd, Leeds
Knipper C, Pichler SL, Rissanen H, Stopp B, Kühn M, Spichtig N et al (2017) What is on the menu in a Celtic town? Iron Age diet reconstructed at Basel-Gasfabrik, Switzerland. Archaeol Anthropol Sci 9:1307–1326. https://doi.org/10.1007/s12520-016-0362-8
Kragten J (1994) Tutorial review. Calculating standard deviations and confidence intervals with a universally applicable spreadsheet technique. Analyst 119:2161–2165
Langston WJ, Chesman BS, Burt GR (2006) Characterisation of European Marine Sites. Mersey Estuary SPA. Marine Biological Association of the United Kingdom. Occasional Publications 18:1–190. https://plymsea.ac.uk/id/eprint/1341/1/Mersey_Estuary_EMS_Final.pdf
Lee-Thorp JA, Sealy JC, van der Merwe NJ (1989) Stable carbon isotope ratio differences between bone collagen and bone apatite, and their relationship to diet. J Archaeol Sci 16:585–599. https://doi.org/10.1016/0305-4403(89)90024-1
Lightfoot E, Pomeroy E, Grant J, O’Connell TC, le Roux P, Zakrzewski S et al (2020) Sea, sickness and cautionary tales: a multi-isotope study from a post-mediaeval hospital at the city-port of Gibraltar (AD 1462–1704). Archaeol Anthropol Sci 12:273. https://doi.org/10.1007/s12520-020-01220-0
Longin R (1971) New method of collagen extraction for radiocarbon dating. Nature 230:241–242. https://doi.org/10.1038/230241a0
MacKenzie L, Speller CF, Holst M, Keefe K, Radini A (2021). Dental calculus in the industrial age: human dental calculus in the post-medieval period, a case study from industrial Manchester. Quat Int [Online]. https://doi.org/10.1016/j.quaint.2021.09.020
Marshall W (1818) The review and abstract of the county reports to the Board of Agriculture. Thomas Wilson & Sons, for Longman, Hurst, Rees, and Orme
Martin JP (2012) Memoir of a revolutionary soldier: the narrative of Joseph Plumb Martin. Dover Publications, USA
Mays SA (1997) Carbon stable isotope ratios in mediaeval and later human skeletons from northern England. J Archaeol Sci 24:561–568. https://doi.org/10.1006/jasc.1996.0139
Mekota A-M, Grupe G, Ufer S, Cuntz U (2006) Serial analysis of stable nitrogen and carbon isotopes in hair: monitoring starvation and recovery phases of patients suffering from anorexia nervosa. Rapid Commun Mass Spectrom 20:1604–1610. https://doi.org/10.1002/rcm.2477
Messinger GS (1985) Manchester in the Victorian age: the half-known city. Manchester University Press Manchester
Metcalfe RS (2015) Meat, commerce and the city: the London food market, 1800–1855. Routledge, UK
Millard A, Blesch J, Scollan M, Rex J, Ulewicz K, Grocke D (2015) Appendix: carbon and nitrogen isotopes in animals from Durham. In: Millard AR, Dodd L, Nowell G (eds) Palace green library excavations 2013 (PGL13): isotopic studies. University of Durham
Minagawa M, Wada E (1984) Stepwise enrichment of 15N along food chains: further evidence and the relation between δ15N and animal age. Geochim Cosmochim Acta 48:1135–1140. https://doi.org/10.1016/0016-7037(84)90204-7
Müldner G, Richards MP (2007) Stable isotope evidence for 1500 years of human diet at the city of York, UK. Am J Phys Anthropol 133:682–697. https://doi.org/10.1002/ajpa.20561
Nash SH, Bersamin A, Kristal AR, Hopkins SE, Church RS, Pasker RL et al (2012) Stable nitrogen and carbon isotope ratios indicate traditional and market food intake in an indigenous circumpolar population. J Nutr 142:84–90. https://doi.org/10.3945/jn.111.147595
Newman S, Holst M (2016). Hazel Grove Osteological Report No: 2116. CFA Archaeology Ltd, Leeds
Newman SL, Gowland RL (2017) Dedicated followers of fashion? Bioarchaeological perspectives on socio-economic status, inequality, and health in urban children from the Industrial Revolution (18th-19th C), England. Int J Osteoarchaeol 27:217–229. https://doi.org/10.1002/oa.2531
Nitsch EK, Humphrey LT, Hedges REM (2010) The effect of parity status on δ15N: looking for the ‘pregnancy effect’ in 18th and 19th century London. J Archaeol Sci 37:3191–3199. https://doi.org/10.1016/j.jas.2010.07.019
Nitsch EK, Humphrey LT, Hedges REM (2011) Using stable isotope analysis to examine the effect of economic change on breastfeeding practices in Spitalfields, London, UK. Am J Phys Anthropol 146:619–628. https://doi.org/10.1002/ajpa.21623
Nitsch EK, Charles M, Bogaard A (2015) Calculating a statistically robust δ13C and δ15N offset for charred cereal and pulse seeds. Sci Technol 1:1–8. https://doi.org/10.1179/2054892315Y.0000000001
North R (1714) A discourse of fish and fish-ponds done by a person of honour. E. Curll, at the Dial and Bible against St. Dunstan’s Church in Fleet-Street, London
O’Brien PK, Quinault R (1993) The Industrial Revolution and British Society. Cambridge University Press, Cambridge
Ó’Gráda C (1989) The Great Irish Famine. Cambridge University Press, Cambridge
Picard L (2006) Victorian London: the tale of a city 1840–1870. Macmillan, USA
Pickering P, Tyrell A (2000) The people’s bread: a history of the anti-corn law league. Bloomsbury Publishing, London
Plumb C (2010) Exotic animals in eighteenth-century Britain. Unpublished doctoral dissertation. University of Manchester: Manchester. http://www.rhinoresourcecenter.com/pdf_files/134/1345701669.pdf. Accessed 4 April 2021
Powers N, Wilson AS, Montgomery J, Bowsher D, Brown T, Beaumont J (2013) No certain roof but the coffin lid: exploring the commercial and academic need for a high level research framework to safeguard the future of the post-medieval burial resource. In: Dalglish C (ed) Archaeology, the public and the recent past. The Boydell Press, Woodbridge, pp 125–144
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 4 April 2021
Redhead W, Laing R, Marshall W, Smellie W et al (1792) Observations on the different breeds of sheep, and the state of sheep farming in some of the principal counties of England. Printed by W. Smellie and sold by W. Creech. http://agris.fao.org/agris-search/search.do?recordID=US201300178636. Accessed 2 May 2020
Registrar-General (1854) Thirteenth Annual Report of the Registrar-General of Births, Deaths and Marriages in England (London)
Richards M (2006) Palaeodietary reconstruction. In: Brickley M, Buteaux S, Adams J, Cherrington R (eds) St. Martin’s uncovered: investigations in the churchyard of St. Martin’s-in-the-Bull Ring, Birmingham, 2001, Oxbow Books, Oxford, pp 147–151
Roberts CA, Cox M (2003) Health and disease in Britain: from prehistory to the present day. Sutton Publishing, Gloucester
Roberts P, Weston S, Wild B, Boston C, Ditchfield P, Shortland AJ et al (2012) The men of Nelson’s navy: a comparative stable isotope dietary study of late 18th century and early 19th century servicemen from Royal Naval Hospital burial grounds at Plymouth and Gosport, England. Am J Phys Anthropol 148:1–10
Roberts P, Fernandes R, Craig OE, Larsen T, Lucquin A, Swift J et al (2018) Calling all archaeologists: guidelines for terminology, methodology, data handling, and reporting when undertaking and reviewing stable isotope applications in archaeology. Rapid Commun Mass Spectrom 32:361–372
Rowntree BS (1901) Poverty: a study of town life. Macmillan, London
Schoeninger MJ (1985) Trophic level effects on 15N/14N and 13C/12C ratios in bone collagen and strontium levels in bone mineral. J Hum Evol 14:515–525. https://doi.org/10.1016/S0047-2484(85)80030-0
Schoeninger MJ, DeNiro MJ (1984) Nitrogen and carbon isotopic composition of bone collagen from marine and terrestrial animals. Geochim Cosmochim Acta 48:625–639. https://doi.org/10.1016/0016-7037(84)90091-7
Schoeninger MJ, DeNiro MJ, Tauber H (1983) Stable nitrogen isotope ratios of bone collagen reflect marine and terrestrial components of prehistoric human diet. Science 220:1381–1383. https://doi.org/10.1126/science.6344217
Schwarcz HP, Schoeninger MJ (2012) Stable isotopes of carbon and nitrogen as tracers for Paleo-diet reconstruction. In: Baskaran M (ed) Handbook of Environmental Isotope Geochemistry: Vol I. Springer Berlin Heidelberg, Berlin Heidelberg, pp 725–742
Scola R (1992) Feeding the Victorian city: the food supply of Manchester, 1770–1870. Manchester University Press, Manchester
Spencer C (2000) The British Isles. In: Kiple KF, Ornelas KC (eds) The Cambridge World History of Food. Cambridge University Press, Cambridge, pp 1217–1226
Stead J (1985) Food and cooking in 18th century Britain: history and recipes. English Heritage, UK
Strohalm M, Kavan D, Novák P, Volný M, Havlíček V (2010) mMass 3: a cross-platform software environment for precise analysis of mass spectrometric data. Anal Chem 82:4648–4651. https://doi.org/10.1021/ac100818g
Szpak P, Metcalfe JZ, Macdonald RA (2017) Best practices for calibrating and reporting stable isotope measurements in archaeology. J Archaeol Sci Rep 13:609–616. https://doi.org/10.1016/j.jasrep.2017.05.007
Trickett MA (2006) A tale of two cities: diet, health and migration in post-medieval Coventry and Chelsea through biographical reconstruction, osteoarchaeology and isotope biogeochemistry. PhD Thesis, Durham University http://etheses.dur.ac.uk/1330/
Trow-Smith R (2013) A history of British livestock husbandry, 1700–1900. Routledge, UK
Turner M J (1995) Reform and respectability: the making of a middle-class liberalism in early 19th-century Manchester: v. 40. (Chetham Society S.). Carnegie Publishing Ltd, UK
Tykot RH, van der Merwe NJ, Hammond N (1996) Stable isotope analysis of bone collagen, bone apatite, and tooth enamel in the reconstruction of human diet. ACS Symp. Ser 625:355–365. https://doi.org/10.1021/bk-1996-0625.ch025
van Klinken GJ (1999) Bone collagen quality indicators for palaeodietary and radiocarbon measurements. J Archaeol Sci 26:687–695. https://doi.org/10.1006/jasc.1998.0385
Walvin J (2017) Sugar: the world corrupted, from slavery to obesity. Hachette, UK (Kindle version)
Waters-Rist AL, de Groot K, Hoogland MLP (2022) Isotopic reconstruction of short to absent breastfeeding in a 19th-century rural Dutch community. PLoS ONE 17(4):e0265821. https://doi.org/10.1371/journal.pone.0265821
Wise MJ, O’Thorpe PN (1950) The growth of Birmingham, 1800–1950. In: Wise MJ (ed) Birmingham in its Regional Setting. British Association for the Advancement of Science, London, pp 213–228
Wise MJ (1951) Essays on the growth of Birmingham and other contributions to the geographical study of the Birmingham District. PhD thesis, University of Birmingham
Wright E, Corbino C, Albarella U (2017) Norton Priory, Runcorn, Cheshire: the animal bones. University of Sheffield, Department of Archaeology
Acknowledgements
Thanks go to CFA Archaeology and Runcorn Development Corporation for giving permission to analyse the human populations studied here. Thanks also go to Jamie Walker from CFA Archaeology and Lynn Smith from Norton Priory Museum and Gardens who gave permission to sample animal skeletal collections. Finally, we would like to thank multiple people from the University of York, James Nottingham (animal bone identification), Krista McGrath (ZooMS), Helen Goodchild (GIS) and especially Matthew von Tersch (mass spectrometry).
Funding
BC was funded by the Arts and Humanities Research Council, AH/L503848/1, and MJC was funded by Danish National Research Foundation, DNRF128. This work was supported by the Arts & Humanities Research Council (grant number AH/L503848/1) through the White Rose College of the Arts & Humanities with analysis subsidised by the Department of Archaeology, University of York.
Author information
Authors and Affiliations
Contributions
BC and MA conceived of the study, BC undertook collagen extractions and analysis. BC wrote the first draft with MA with revisions, contributions and comments from all authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chidimuro, B., Holst, M., Newman, S. et al. Grim up North? Exploring the diet of urban populations in post-medieval Greater Manchester, England, using stable isotope analysis. Archaeol Anthropol Sci 15, 174 (2023). https://doi.org/10.1007/s12520-023-01881-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12520-023-01881-7