Abstract
Debate over migrations to Britain during the fifth and sixth centuries AD is still rampant in archaeological discourse. Stable carbon, nitrogen and oxygen isotope values from multiple tissues in individuals buried at Finglesham in Kent during the first millennium AD demonstrate not only migration of individuals to the region but also highlight community integration through foodways and refute previous models of ‘invasion’ and replacement. This case study community suggests gendered differences in mobility into early medieval England, with males more likely to be migrants from cooler regions than women. It also challenges traditional narratives of social status in these furnished cemeteries being linked to diet or migrant status with no clear correlations found between funerary treatment and isotopic signatures. This multi-tissue and multi-isotope study tracks dietary changes in this multi-origin community throughout their lives and shows that they may have even changed their diets to adapt to Christianising influences in the region.
Similar content being viewed by others
Introduction
Combining isotopic signatures from different tissues gives powerful insight into patterns of diet and mobility throughout the life course. This paper combines carbonate signatures from human tooth enamel, with carbon and nitrogen from bone and dentinal collagen of the same individuals to investigate how one community in Kent changed and adapted during the highly dynamic period of the fifth to eighth centuries AD.
The period covered here spans the ‘Adventus Saxonum’ (or ‘coming of the Saxons’) (Hills 2013b, 2015; Michelet 2017), the Late Antique Little Ice Age (c. 536 to 660AD, LALIA) (Büntgen et al. 2016, 2017; Di Cosmo et al. 2017; Fei et al. 2007; Helama et al. 2017; Loveluck et al. 2018; Luterbacher et al. 2016), the Plague of Justinian (c. 541–750 AD) (Keller et al. 2019; Mordechai et al. 2019), and the Christian mission of St Augustine to Kent (597 AD). It is therefore not surprising that there were major cultural and socio-economic changes during this period, namely changes in burial practice and grave goods, farming practices and trade, although the relationships between all factors is highly complex. The impact and scale of these events are much debated, the ‘Adventus’ in particular, and other isotopic studies claim to show evidence for contemporary migrant populations to those proposed here (Hughes et al. 2014, 2018).
The ‘Adventus’ theoretically occurred at the end of the Roman occupation in Britain in the fifth century AD and resulted in large material-cultural changes in England, particularly in funerary rites and grave goods. Cemeteries in Kent are frequently cited as key evidence in the debate around the ‘Adventus Saxonum’ due to distinctive female dress-styles which are seen by some as definitive proof of incoming groups (Brookes and Harrington 2010; Härke 2011; Richardson 2005; Sorensen 1999). The main historical sources for this migration are Bede, Gildas and the Anglo-Saxon Chronicle which all state that groups of people migrated from parts of what is now the Netherlands, northern Germany and the Jutland peninsula to Britain (Gildas 2010; The Venerable Bede 2009; Unknown 2008).Footnote 1 Scholarship on post-Roman migration(s), particularly to England, has shifted between elite replacement models with top-down cultural change, models of large-scale migration over several generations and anti-migrationist thought from the 1990s onwards (Hamerow 1997; Härke 2003, 2004, 2011; Hills 2013a, 2013b, 2015; Pattison 2008; Thomas et al. 2006, 2008).
Previous isotopic studies on early medieval communities have shown that there was likely a large amount of movement around Europe throughout the first millennium AD but identifying ‘non-locals’ and outliers in datasets still proves difficult (Brettell et al. 2012a, b; Evans et al. 2012; Groves et al. 2013; Hakenbeck 2013; Hemer et al. 2013; Krzewińska et al. 2018). However, taking a life course approach with isotopic data to investigate not only if a cemetery includes migrants, but their cultural integration via foodways, alongside grave goods and other factors is as yet underutilised in early medieval cemeteries, especially in England. This is the largest study utilising multi-tissue multi-isotope analyses for any single cemetery in early medieval England and therefore goes a long way towards a better understanding of the nature and impact of early medieval migrations in England in post-Roman Britain, and other major transitions, like Christianisation.
Background
Location and early medieval archaeology of Finglesham and its surrounds
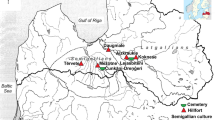
Finglesham is located in eastern Kent (51° 14′ 5.9994″, 1° 20′ 24″; see .
Figure 1) at approximately 12 m above sea level on a mixture of Cretaceous chalk, Palaeogene alluvium, sand, gravel, silt, and diamicton (British Geological Survey n.d.; Chadwick Hawkes and Grainger 2006). The δ18OMAP (modelled annual precipitation) value calculated for the site using the OIPC (online isotopes in precipitation calculator) is − 6.9‰ (Bowen 2019; Bowen and Revenaugh 2003; Leggett et al. 2021; Lightfoot and O’Connell 2016).
Kent is well placed for cross-channel and North Sea trade, with several wics/emporia established by the eighth century, including Rochester, Sarre, Fordwich, Sandwich, Dover and Sandtun (Brookes and Harrington 2010: 83; Hill and Cowie 2001; Leggett 2017; Pestell 2011). Finglesham is close to several of these trading ports, and their limbs (see .
Figure 1) so therefore was likely well linked into coastal trade which may have facilitated migration of people as well as the importation of luxury and foreign goods found in some of the graves (Brookes and Harrington 2010; Chadwick Hawkes and Grainger 2006; Soulat 2013, 2018).
Grave goods of Frankish origin are not uncommon in Kent but are almost absent in other parts of England, suggesting that Kent and Francia had special trade relationships in the early middle ages (Brookes and Harrington 2010: 46–47; Chadwick Hawkes and Grainger 2006; Sorensen 1999; Soulat 2013, 2018). This unique cross-channel relationship also included royal marriages such as King Æthelberht of Kent’s marriage to the Frankish princess Bertha; this alliance is what is often credited as the conduit for the Christian conversion of the ‘English’ in the late sixth and early seventh century (at least by Bede) (Stenton 1971: 59–60; Blair 2003: 27–28, 116–117; The Venerable Bede 2009: 39–41; Brookes and Harrington 2010: 44,69–70).
In the seventh and eighth centuries, a large shift occurs in funerary practices across western Europe, generally termed the ‘final phase’ of furnished burial (Brownlee 2020; Welch 2011). Finglesham is one of the cemeteries where these shifts can be seen. It, like other cemeteries in Kent, sees extremely elaborate graves with large grave markings and barrows (elite female graves being especially well furnished) appear alongside an increasing number of individuals with little to no grave goods. However, compared with the rest of England furnished burial continues later in Kent (Brownlee 2019: 185).
These marked shifts in funerary displays are also coupled with large differences in stature between women and men in the fifth/sixth centuries which seem to disappear by the seventh/eighth (Richardson 2005: 100–102, 252). This is used by Richardson and Härke (2011) to argue for men and women coming from different ethnic groups during the ‘Adventus’, but it could of course be due to a number of other factors such as differences in nutrition, as investigated further below.
Kent’s particularly well recorded processes of religious conversion and cross-cultural contact during the early medieval period make it somewhere we would expect to find individuals of mixed childhood origins, and see the impact of migration, religious conversion, trade and exchange on foodways. Consequently, Finglesham, as a large fifth to eighth century cemetery, close to the coast is an exciting case study through which these cultural shifts can be explored.
Finglesham was first excavated by Stebbing and Whiting in 1928–1929, with further excavations by Sonia Chadwick Hawkes between 1959 and 1967. Hawkes excavated 216 inhumation graves, 201 of which were osteologically analysed (Chadwick Hawkes and Grainger 2006). Forty-six individuals were selected for primary isotopic analyses (see graves highlighted in Fig. 2).
Site plan of the early medieval cemetery at Finglesham, Kent, with burials sampled for stable isotope analyses filled with stripes, and unsampled graves in solid grey, redrawn by S. Leggett from Chadwick Hawkes and Grainger (2006)
The cemetery was in use from approximately the fifth to the eighth century AD and seems to represent a ‘normal’ burial population in its age and sex profiles (Chadwick Hawkes and Grainger 2006: 15, 324). The majority of burials are aligned W-E with heads to the west, but there are some variations on this, as seen in Fig. 2. The site also boasts many tumuli/barrows and graves with post holes and other wooden structures alongside plain earth-cut graves.
The choice of Finglesham as a case study cemetery for the region was clear—it has the largest sample size for all three tissues (bone, dentine and enamel) in Kent to date (Leggett et al. 2021). This enabled more robust analyses which would not be possible for cemeteries with fewer individuals, or with only one tissue analysed. It is also a well-known, well-excavated and well-documented cemetery that has a good balance between female and male graves and variation in levels of grave provisioning so as to not overly bias the dataset with unbalanced sample sizes between social or biological groups (e.g., more men than women, more richly furnished individuals. See ‘Statistical analyses’ section below for implications).
Stable isotope analyses
Chemical techniques are now relatively commonplace in bioarchaeological studies which investigate diet and mobility in the past. There is a growing trend for multi-tissue and multi-isotope studies at individual and populational levels (Bond et al. 2016; Gregoricka et al. 2017; Lamb et al. 2012; Ryan et al. 2018; Zhu and Sealy 2019).
Quantifiable isotopic variations in water (caused by climate), geologies and different kinds of food and drink underpin stable isotope methodologies (Gannes et al. 1998). Due to differences in tissue turnover rates, different bones reflect the average isotopic composition of food and drink consumed, and the environment these foods were sourced from as those tissues were being formed and re-modelled throughout an individual’s life (Hedges et al. 2007; Pederzani and Britton 2019). Similarly teeth (which do not remodel during life) reflect the period of consumption and environmental conditions during their formation (Beaumont et al. 2013; Pederzani and Britton 2019). Differences in the environmental variation of different isotopes and dietary routing into various bodily tissues are briefly explained below.
Collagen (bone and dentine)—carbon and nitrogen
The use of stable carbon and nitrogen isotopes for dietary reconstruction in archaeology is a well-established technique (Ambrose 1986; Bocherens and Drucker 2003; Kellner and Schoeninger 2007).
Carbon stable isotope ratios can discern between diets based on C3 plants (e.g. most vegetables, wheat, barley, oats, rye), C4 (mainly drought adapted grasses, e.g. millet, sorghum, amaranth) and CAM (e.g. succulents and cacti) plants due to differences in their photosynthetic pathways causing isotopic fractionation (Farquhar et al. 1989; Hoefs 2018: 63–65; O’Leary 1988). In early medieval England (where we do not expect to see any C4 or CAM plant consumption (Alt et al. 2014; Ganzarolli et al. 2018; Hagen 2006: 23, 33, 38–39; Hakenbeck et al. 2017)), they are also useful in differentiating between marine versus terrestrial food resources (O’Leary 1988). Nitrogen stable isotope ratios indicate trophic level and are generally assumed to increase ~ 3–5‰ per level of the food chain, with controlled diet studies suggesting diet-collagen spacing can be as high as ~ 6‰ (Ambrose 1986, 1991; Bocherens and Drucker 2003; Hedges and Reynard 2007; O’Connell et al. 2012). They are therefore useful in indicating plant versus animal protein intake, as well as consumption of marine and freshwater resources when combined with carbon isotope values (Bocherens and Drucker 2003; Lucy et al. 2009). δ15 N in humans and animals can also be altered by factors such as breastfeeding/weaning, and pregnancy/lactation (Beaumont et al. 2015; Fuller et al. 2005, 2006; Haydock et al. 2013; Richards et al. 2002).
Collagen in bone and dentine is composed of both essential and non-essential amino acids, and thus reflects diet but also other metabolic processes and nutrient pools in the body. The carbon and nitrogen in collagen derives primarily from dietary protein but nitrogen in particular can be impacted significantly by periods of nutritional stress, and carbon in collagen can also be drawn from carbohydrates and fats which can be from the consumer’s own body (France and Owsley 2015; Fuller et al. 2005; Hedges 2003; Lee-Thorp et al. 1989; Makarewicz and Sealy 2015; O’Connell et al. 2012).
Due to differences in structure between trabecular and compact bone it is assumed that trabecular bone (e.g. majority of rib bone and hence its use in this study) has a much faster tdurnover rate and thus represents a shorter window for diet, while long bones largely made of compact bone have far longer turnover periods; however exact rates are still elusive (Hedges et al. 2007; Sealy et al. 1995; Smith and Rennie 2007). Unlike bone, dentine usually does not remodel once formed, so turnover rates are not a concern in the same way, however, dietary routing and changes to metabolism (e.g. stress events) are. Dentine reflects diet during the period of tooth root formation which begins after completion of the crown, usually a snapshot of 1–3 years depending on the tooth selected (Scheid 2007: 328).
Bioapatite—oxygen and carbon
Bioapatite is the mineral portion of biologically formed tissues, here analysed from tooth enamel carbonate. Enamel, like dentine, does not remodel during life. Therefore, it represents a fixed point in early life (often an average over a few years depending on the tooth selected) for the incorporation of those elements into the tissue matrix. Hence it is a useful tool for mobility and diet studies (Brown and Brown 2011: 100–101; Chenery et al. 2012; Evans et al. 2012; Pederzani and Britton 2019; Price and Burton 2012: 91–92, 95–98).
Slightly different to the routing of carbon in collagen, the carbon in bioapatite is derived from blood bicarbonate, which in turn derives from respiration and therefore is thought to represent whole diet (Ambrose and Norr 1993; Hedges 2003; Howland et al. 2003; Passey et al. 2005; Tieszen and Fagre 1993; Zhu and Sealy 2019). Enamel carbonate therefore gives us a window into whole diet in childhood for the period of crown formation and mineralisation. This is a useful tool to combine with the largely protein-derived δ13C values of collagen by creating bioapatite-collagen offsets. Tissue offsets are however not straightforward, especially in omnivores (i.e. humans). A recent feeding study on pigs suggests that the introduction of marine resources into the diet can substantially change nutrient pooling and dietary routing of amino acids and thus change the ∆13Cwhole diet-tissue relationship that holds well in terrestrial contexts (Webb et al. 2017). However, Webb et al. (2017) did not collect enamel carbonate samples in their study, so it is hard to interpret how this plays out in bioapatite versus proteinaceous tissues (collagen) to attempt corrections and better understand the whole diet-apatite relationship in omnivores. Although their work does suggest that ∆13Cbioapatite-collagen values in humans (and other animals) may not always reflect trophic level, looking at a combination of the ecological literature and work done on Jomon period Japanese populations, suggests that ∆13Cbioapatite-collagen values can give a suggestion of trophic-like relationships, in particular for disentangling proportions of mixed marine/terrestrial/freshwater protein discrimination (especially in otherwise C3 environments) (Clementz et al. 2007, 2009; Clementz and Koch 2001; Kusaka 2019; Kusaka et al. 2015). Although caution is needed as more feeding studies and work on modern populations (both animals and people) is needed to better understand underlying dietary routing and isotopic composition of all portions of foodstuffs.
The δ18O values of bioapatite are determined by the isotopic composition of body water in mammals, which is derived from consumed oxygen sources such as inhaled air, drinking water and oxygen components of food (structural oxygen and food water), in a mass balance with excretions (e.g. water vapour through breath or sweat, water in waste products and exhaled carbon dioxide) (Pederzani and Britton 2019).
An extensive review of archaeological applications of isotope analyses using oxygen and the underlying principles has been recently published by Pederzani and Britton (2019). Variation in δ18O baselines is due to the hydrological cycle where water is continuously rained out and evaporated. During this cycle, proximity to coast (continentality), seasonality, temperature, altitude, amount of rainfall and humidity all play a role in δ18O variability (Bowen and Revenaugh 2003; Lightfoot and O’Connell 2016; Pederzani and Britton 2019; Rozanski et al. 1992, 2013). Thus, δ18O values vary in relatively predictable patterns geospatially and climatically. δ18O fractionation is largely driven in natural systems by evaporation, whereby the preferential evaporation of the lighter to the heavier isotope, leaves bodies of water which are either large, or in hot climates, enriched in 18O (Bowen and Revenaugh 2003; Iacumin et al. 1996; Longinelli 1984; Pederzani and Britton 2019; Rozanski et al. 1992, 2013).
Cooking, brewing and stewing
Humans are unique in their consumption of artificially altered beverages and foods in high abundances. The ‘brewing and stewing’ effect is a confounding factor in some archaeological stable isotope studies using oxygen and is difficult to correct for (Brettell et al. 2012a, b; Pederzani and Britton 2019).
The heating processes involved in making drinks such as teas or worts, alcoholic distillation or stewing cause liquid evaporation which leaves the product enriched in the heavier isotope (Brettell et al. 2012a, b; Pederzani and Britton 2019). Mammalian milk has also been shown to be enriched compared to body water and drinking water values (Lin et al. 2003; Pederzani and Britton 2019). Brettell et al. (2012a, b) estimated that δ18O values may be enriched by as much as + 2.3‰ or possibly more, given the historical and archaeological evidence. In essence, enriched 18O values can point to foodways which involve a significant amount of brewing and stewing as shown with King Richard III (Brettell et al. 2012a, b; Lamb et al. 2014; Pederzani and Britton 2019).
Materials and methods
Materials
As per Leggett et al. (2021) selection of individuals was based on skeletal preservation as initially assessed from site reports as well as the condition of the skeletons at the point of sampling in the Duckworth Laboratory collection. Where possible, individuals with both suitable ribs and surviving teeth were preferred. Adults were preferentially sampled with some late sub-adults being chosen due to cultural perceptions of adulthood in the Early Middle Ages with adulthood, at least in part, starting in the mid-to-late teens as seen life stages marked by different grave assemblages which is reflected in their age categories assigned here (Crawford 2018; Leggett et al. 2021; Stoodley 2011). Good bone and enamel preservation were also key factors for selection. Individuals whose bones might have low collagen yields or tissues otherwise affected by diagenesis, and thus might complicate the interpretation of results, were avoided. This was assessed visually and physically by the texture and appearance of the bone (e.g. was it crumbling or discoloured, were there significant signs of microbial activity, were there any signs of petrification in the bones, etc.). After preservation and availability of skeletal elements, sampling as evenly as possible spatially across the site (see Fig. 2) with roughly equal numbers of females and males was attempted. Both males and females were included irrespective of grave provisioning so long as they met the above preservation criteria to avoid sampling based purely on richness of graves or the skewness this brings to both chronology and gendering of graves (Bayliss et al. 2013; Brownlee 2019; Leggett et al. 2021). This has meant a mixture of furnished and unfurnished graves, with adults largely at life stages 4/5, dating primarily to phases B–D, but more radiocarbon dating on this site is forthcoming (see Table 1 and Leggett et al. 2021 for a breakdown of individuals and their categorisations).
Ribs were preferred for bone specimens (Hedges et al. 2007; Lucy et al. 2009). Permanent second premolars or second molars were preferred due to their similarity in development timings and because they represent a post-weaning signature in both enamel and dentine (Beaumont et al. 2015; Evans et al. 2012; Scheid 2007: 326–334). Some individuals did not have teeth suitable for sampling (e.g. could not easily extract the tooth, or too carious or worn on inspection), but the priority was to limit damage to skeletons, so if a tooth could not be easily extracted, only bone was taken.
Of the 46 individuals sampled for stable isotope analyses, 45 individuals had rib fragments available for analysis (Table 1). Forty of those individuals had loose teeth or teeth loose enough to extract without damaging the maxilla/mandible to analyse both dentine and enamel from the same tooth. Forty of the 46 individuals analysed have more than one tissue with isotopic data, and 38 have all three tissues with data included here as summarised in Table 1.
Individuals sampled range from date category B (450–580 AD) to E (690–790 AD), with more individuals from periods C/D (c. 580–690 AD) and D (630–690 AD), and slightly more males than females in the sample. The phasing of these individuals was based on assessments in the original excavation report and subsequent radiocarbon dating (Bayliss et al. 2013; Chadwick Hawkes and Grainger 2006; Leggett et al. 2021). Full details on phasing can be found in the data paper by Leggett et al. (2021).
Forty-one of the 46 individuals had skeletal stature estimates available, and these range between 145 and 183 cm (Chadwick Hawkes and Grainger 2006); female skeletons range from 145 to 169 cm, and males 165–183 cm which agrees with the analysis of Kentish stature more broadly undertaken by Richardson (2005: 100–102, 252). Numbers of grave goods within the Finglesham subsample range from 0 to 13, with a maximum of two foreign items (as specified in the original report) in one grave (gr 57).
Methods
All samples were prepared in the McDonald Institute for Archaeological Research, University of Cambridge and analysed on mass spectrometers housed in the Department of Earth Sciences, University of Cambridge. Full details on laboratory methods and protocols used, as well as the collation and standardisation of site context data and funerary practices are detailed in the metadata in Leggett et al. (2021), where the data are also fully accessible. The laboratory methods used are therefore briefly outlined below.
Bioapatite—enamel carbonate isotopic analyses
As detailed in Leggett et al (2021), tooth enamel powder was prepared for stable isotope analysis of bioapatite (carbonate) following the Balasse method (Balasse et al. 2002). Treated enamel powder was analysed in single aliquots at the Godwin Laboratory, Department of Earth Sciences, University of Cambridge, using a Gas Bench II coupled to a Delta V mass spectrometer for isotopic analysis. The enamel carbonate samples are reported with reference to the VPDB standard, calibrated through the NBS19 international standard for carbon and VSMOW (Vienna Standard Mean Ocean Water) for oxygen and analytical error for the carbonate samples is ± 0.08‰ for δ13C and ± 0.10‰ for δ18O as calculated through these international standards and in-house Dorothy Garrod Laboratory standards of homogenised prehistoric animal tooth enamel called standard 2 (δ18O − 4.9, δ13C − 13.0) and 3 (δ18O − 1.1, δ13C − 10.0).
As detailed in Leggett et al. (2021), there are many different conversion and correction equations for oxygen isotope values from human tooth enamel; from carbonate to phosphate, from phosphate to drinking water, drinking water to temperature… and there is still much debate in the field surrounding this (Lightfoot and O’Connell 2016; Pederzani and Britton 2019). As the enamel here was analysed as carbonate, a variety of phosphate and drinking water conversions were calculated and reported in Leggett et al. (2021). The original carbonate δ18OPDB measurements are reported here alongside δ18OPO4 VSMOW (phosphate) values converted from δ18OPDB using the Chenery et al. (2012) equation.
Δ18Odw-MAP was also calculated for each sample from δ18Odw calculated from δ18Ocarb VSMOW (δ18Odw = 1.59*δ18Ocarb VSMOW—48.634) and the site MAP value given by the OIPC mentioned above (− 6.9‰) (Bowen 2019; Bowen and Revenaugh 2003; Chenery et al. 2012; Leggett et al. 2021). This was done as a simplistic measure to complement traditional visual and statistical methods for the identification of outliers/migrants using δ18O values by calculating the difference between the theoretical δ18OMAP values and the δ18Odw values calculated from human tooth enamel. The equation is extremely simple:
Δ18Odw-MAP = δ18Odw—δ18OMAP.
This difference can be used as a guide to how much an individual’s δ18Odw value deviates from their place of burial. Given the maximum estimated inter-laboratory and population variability is ± 2‰ for δ18O, individuals with values within ± 2‰ should be regarded as ‘local’ (Jay 2005; Lightfoot and O’Connell 2016; Pederzani and Britton 2019; Pestle et al. 2014; White et al. 2002, 2004). Values above + 2‰ could be migrants but should have ‘brewing and stewing’ fractionation factors considered as part of interpretation, however those below − 2‰ are likely real migrants or people who lived during a climatic cooling event (such as LALIA) since there are not any known cultural processes which can alter δ18O values in this direction (Brettell et al. 2012a, b; Pederzani and Britton 2019).
Collagen—bone and dentine carbon and nitrogen isotopic analyses
Collagen was extracted from bone and dentine samples following a modified Longin method (Leggett et al. 2021; Longin 1971; Privat et al. 2002; Richards and Hedges 1999). Aliquots of collagen were run in triplicate using an automated elemental analyser coupled in continuous-flow mode to an isotope-ratio-monitoring mass-spectrometer. Stable isotope values of bone collagen and dentine are reported relative to an internationally defined scale—VPDB (δ13C) and AIR (δ15N). Analytical error (1σ) for all collagen samples is ± 0.20‰. As per the companion data paper Leggett et al. (2021) the standards used are IAEA standard of caffeine for carbon and nitrogen (δ15N + 1.0/1.1‰, δ13C − 27.5‰); in-house standards of nylon (δ15N − 3.14‰, δ13C − 26.55‰), alanine (δ15N − 1.4‰, δ13C − 26.9‰), ‘new’ alanine (δ15N − 1.22‰, δ13C − 23.88‰), protein 2 (δ15N + 6.0‰, δ13C − 26.9/ − 27.0‰) and EMC (Elemental Microanalysis Caffeine, δ15N − 2.5/ − 2.6‰, δ13C − 35.8/ − 35.9‰) for carbon, nitrogen and atomic C/N ratios.
Statistical analyses
Archaeological data often have large biases (e.g. large differences in sample sizes between groups) and these biases would lead to the violation of assumptions for common statistical approaches (e.g. assumption of equality of variance across groups) (Zuur et al. 2010). Furthermore, the sampling design was nested with varying numbers of sexes, genders and age groups as well as funerary treatments and date categories represented. Frequentist approaches tend to perform poorly with these kinds of unbalanced data structures. However, Bayesian and exploratory data analysis (EDA) get around some of these issues.
EDA is a state of flexibility which does not require statistical significance or probability, and therefore avoids the proliferation of type I and type II errors and other such problems, reducing the likelihood of making false archaeological interpretations (Tukey 1977: 794, 806; Zuur et al. 2010). On this vein, Bayesian thinking is preferable to a frequentist approach as it too is more flexible; it can take on non-parametric data and uncertainty without having to violate assumptions and commit statistical sins (Kruschke 2013, 2014; Kruschke and Liddell 2018; Lavine 2019; McElreath 2018: 2–4). Therefore, a Tukey-style EDA framework and Bayesian thinking was adopted in analyses. Data were explored graphically first before undertaking statistical analyses where appropriate (McElreath 2018; Tukey 1977; Zuur et al. 2010). This can be broadly summarised as ‘New Statistics’ which is underscored by using Open Science practices, embracing both explorative and planned statistical methods (Calin-Jageman and Cumming 2019; Collaboration 2015; Kruschke and Liddell 2018). Furthermore, no p-values will be reported here as per the American Statistical Association (ASA) guidelines (Wasserstein et al. 2019; Wasserstein and Lazar 2016).
Statistical analyses and graphics were conducted using Free and Open Source R version 4.0.4 and Rstudio version 1.4.1106 (R Development Core Team 2021; RStudio Team 2021). The code is freely available as part of the Supplementary Material ESM1, with data primarily drawn from Leggett et al. (2021), with additional and re-formatted data given in the article and in ESM2. Maps were created using the Free and Open Source QGIS version 3.10 unless otherwise attributed (QGIS 2020).
Data were visualised and explored with a range of plots including scatterplots, violin plots, and bagplots using R packages ‘ggplot2’, ‘ggridges’, ‘ggExtra’ and the geom_bag function (Attali and Baker 2019; Marwick 2018; Wickham 2019; Wilke 2020).
Bagplots are bivariate versions of boxplots. They show the depth median (the point with the highest possible Tukey depth) which is roughly analogous to the univariate median and visualised as a cross (e.g. Figure 7). Then, there is a darker shaded polygon around the cross that encloses 50% of the points around the depth median called the ‘bag’, analogous with the box in a box plot. Around the bag is another lighter polygon which is three-times the size of the bag called the ‘loop’, which is equivalent to the whiskers on a box plot. Any points outside the bag and loop are considered outliers and not automatically visualised (Marwick 2018; Rousseeuw et al. 1999; Wickham and Stryjewski 2012).
BEST (Bayesian Estimation Supersedes the t-test) tests were used as a Bayesian alternative to a student’s t-test, to compare between two groups (Kruschke 2013, 2014). The BEST test uses Markov-chain Monte-Carlo (MCMC) sampling to generate posterior predictive distributions for group data. In Bayesian statistics posterior predictive distributions (PPDs), and therefore the means of these distributions, are distributions of possible unobserved values which have been predicted based on the observed or ‘real’ data put into the model (Kruschke 2013, 2014). An important point is that the results are drawn from the PPD generated from the MCMC and not the inputted data directly. These were run with R package ‘BEST’ (Kruschke 2013). The outputs of these tests can be found in ESM3 along with an infographic and additional guidance on how to interpret them.
The DetectingDeviatingCells (DDC) algorithm was used to detect outlying cells in the numeric data available for the Finglesham burials (i.e. isotopic values, stature, number of grave goods and grave orientation). This was done using the R package ‘cellWise’ (Raymaekers et al. 2020; Rousseeuw and Bossche 2018). DDC can only be used on numeric data and excludes observations with over 50% missing data, and variables which have low variation from the mean. DDC uses the entirety of the data matrix to determine if cells are higher or lower than expected given the rest of the dataset. It therefore tells us if certain individual burials are outliers and for which specific metrics, given the data for the rest of the cemetery (in this case). It produces a graph called a cell map (see Fig. 12) where red cells are higher than predicted, blue cells are lower, yellow is ‘normal’, with orange and purple forming a scale between the extremes. White cells indicate missing values. Black/grey dots next to the row (here columns, as the cell map is rotated for easier viewing) highlight individuals which have half or more cells flagged as outliers (at either end of the scale).
Results and discussion
The data as reported in Leggett et al. (2021) are summarised in Table 1 with additional quality indicators reported (%C, %N and % collagen yield for bone and dentine). Collagen preservation was good across the site (see Table 1), with all samples except the bone collagen from grave 18 meeting the established standards for collagen preservation, and many having preservation well within the range of modern samples (van Klinken 1999).
The δ13C of the bone collagen samples range from − 20.9 to − 19.7‰, and δ15 N ratios of the same samples vary from 8.4 to 10.9‰, with respective means of − 20.1± 0.2‰ and 9.4 ±0.6‰. Dentine from the same individuals has δ13C values from − 20.4 to − 19.3‰ (mean − 19.9 ±0.3‰), and δ15 N values of 7.8–12.6‰ (mean 10.0 ±1.3‰). The δ13Ccarb values range from − 15.4 to − 12.7‰ with an average of − 13.9 ±0.7‰. The δ18OPDB values from Finglesham range from − 6.8 to − 2.9‰, with a mean of − 5.3 ±1.0‰.
Figure 3 shows the enamel δ13Ccarb and δ18Ophosphate results for the cemetery, with multi-modality shown at the site, particularly in δ18Ophosphate values. The split in δ18Ophosphate values occurs around 17.0–17.5‰, with values below 17.0‰ (n = 29) being more numerous than those above (n = 11), which might indicate a mixed-origin group drawing from at least two different water sources. This split in ∆18Odw-MAP values equates to 11 individuals with local or 18O enrichment (possible brewing/stewing) and the other 29 with 18O depletion (see Table 1). This suggests that two thirds of the analysed burials at Finglesham could have childhood origins in colder climates, such as northern Europe.
Despite this marked bimodality in enamel carbonate signatures, Fig. 4 and Fig. 5 demonstrate the relative homogeneity in dietary signatures in both bone and dentine collagen at Finglesham. However, this does not necessarily equate to homogeneity through the life course as indicated by the large dentine δ15 N standard deviation mentioned above.
For bone, Finglesham δ13Ccoll values have a range of 1.2‰, and δ15Ncoll values of 2.5‰ (as mentioned above). The ranges for dentine are similarly tight with δ13Ccoll having a range of 1.1‰, and δ15Ncoll 4.8‰. There is little variation in δ13Ccoll values for either tissue, but δ15Ncoll values in bone suggest at least two different trophic levels in the population. δ15Ncoll values in dentine show more variation which could be due to variability in the types of teeth analysed, although these do not appear to be systematically offset in anyway (predominantly PM2 and M2 but some other teeth were analysed, see Table 1). This is possibly due to the diversity of childhood origins in the population as suggested by the carbonate data above. Overall, the group has a C3 based diet with little variation in the population, with no clear evidence for a substantial marine input into the diet.
There are no clear outliers in the Finglesham subsample identifiable in either Fig. 4 or Fig. 5. This is a population with mixed origins whose dietary habits throughout life are relatively similar to one another. This is not unexpected for a smaller community buried together, where we might expect more localised patterns of consumption on a small scale, especially in bone which is more likely to represent some or all of their time in and around Finglesham and its associated settlement(s).
In the following sections chronological change in migration, dietary change over the life course and tissue offsets, and isotopic differences between the sexes are considered before using the DDC algorithm to identify outliers statistically in the Finglesham population.
Chronological change
Due to the low variation within the δ13Ccoll and δ15Ncoll values for bone and dentine in Finglesham, the relatively short time span the sampled burials represent and the skewed sampling towards those from date categories C and D, diachronic change for diet at the site was not explored. However, change through time in ∆18Odw-MAP values at the site was investigated to see if any particular migration waves are identifiable. Since this involved multiple group comparisons BEST tests were not appropriate here, so a visually comparative EDA approach was used instead. Figure 6 shows that even despite some small sample sizes for earlier dated graves, individuals with negative ∆18Odw-MAP values are present throughout the use of the cemetery. There are only a few individuals with positive ∆18Odw-MAP values, one in date category B/C and the others from c. 580 AD onwards. This shows that Finglesham is a community of migrant individuals to Kent in the earlier centuries from somewhere further north in Europe (probably Scandinavia). The later more ‘local’ and possible ‘brewing and stewing’ signatures which appear one or two generations after the founding of the burial ground are therefore likely the locally born descendants of the original group. This picture could change with more individuals included from the earlier graves, and there may be this bimodality in the earlier phases too, in which case Finglesham would be a fully mixed local and migrant burial community from its inception. There are three individuals whose ∆18Odw-MAP values are 1.9, 1.9 and 2.6‰. For these individuals ‘brewing and stewing’ effects or possible migration from somewhere further south in Europe cannot be ruled out, but without significantly different diets or strontium data it is impossible to say more.
Violin plots of Finglesham \(\Delta\) 18Odw-MAP (Chenery) values through time from c.450 to 790 AD (B = c. 450–580AD, E = c. 690–790AD; see Leggett et al. 2021 for details on chronological designations)
Change through the life course
Bone and dentine δ13Ccoll and δ15Ncoll values from individuals with both tissues analysed in Finglesham were compared, the results of which can be seen in Fig. 7. As with the bone and dentine scatterplots above, the ranges for δ13Ccoll are relatively small for both tissues (<2‰); however, the δ15Ncoll values in dentine are markedly higher. The degree of overlap between the bags and loops, and the proximity of their depth medians is reflected in the results of BEST tests comparing δ13Ccoll and δ15Ncoll values between the tissues which found that they were very similar, with the starkest difference being the standard deviation for dentine δ15Ncoll (see supplementary material).
The isotopic niche for the Finglesham community in earlier life seen in dentine is far broader than that represented by bone, which is interesting, when considering the formation times for the tissues, and the fact that bone remodels. We may have expected the opposite since bone should reflect a larger time frame which could therefore have far more dietary variability. This is likely due to the high degree of mobility seen in the population. Therefore, the dentine represents C3 diets from a variety of childhood origins. Whilst similar to the adult diets seen in bone, it has a broader isotopic niche due to the variety of environments these tissues were formed in during earlier life.
Figure 8 uses bone, dentine and enamel isotopic data to look at trophic position and tissue offsets/enrichment through the life course. There is remarkable consistency over the life course in δ13Ccoll values, with very little change seen in Fig. 8C, although the same graph shows there is more diversity in ∆15Ndentine-bone in the population, which is as expected given the differences between the bag plots of bone and dentine discussed above. ∆13Ccarb-dentine values shown in Fig. 8A, B and D show the majority of individuals at Finglesham sitting around 6–7‰. This means that most of the sampled individuals at Finglesham have ∆13Ccarb-dentine values comparative to terrestrial and freshwater consumers on a scale from herbivory to omnivory (6‰ and up), with six individuals whose values indicate a higher trophic level (∆13Ccarb-dentine values closer to 4‰) (Clementz et al. 2007, 2009; Clementz and Koch 2001). Figure 8A demonstrates that these higher trophic positions (~ 5‰ or below) do not correlate directly to δ15Ncoll values, but what Fig. 8B implies is that in C3 diets, lower ∆13Ccarb-dentine values correlate with low δ13Ccarb values. This indicates that higher trophic levels in individuals may be due to freshwater resources rather than terrestrial or marine protein.
Figure 8 highlights the variability in childhood δ15Ncoll values compared to the signatures in bone, and the C3 nature of diets of people at Finglesham. Very few individuals had protein-heavy diets, those which do are a minority, and this may be from freshwater resources. Since high δ15Ncoll values do not correspond with high trophic levels, as measured through tissue offsets, at Finglesham despite the faunal evidence from (high status sites) such as Lyminge (Knapp 2018), we need concerted efforts to form more appropriate faunal baselines for sites, and consider what other aspects of agriculture and diet may be at play such as plant values (Hamerow et al. 2020).
Sex-based differences
Given the stark differences in height at Finglesham between females and males, and the assertion by Härke and Richardson that this may reflect different communities of origin for males and females (Härke 2011; Richardson 2005), mobility or dietary differences between the sexes on this smaller community scale were tested.
Figure 9 compares ∆18Odw-MAP values between females and males, and, whilst bimodality is found in both groups, it is more evenly split in females who also have the highest values for the site. Both groups are skewed towards negative values and males have the lowest ∆18Odw-MAP values for the subsample. A BEST test showed no difference between the groups, however since this is largely based on means, it is perhaps not the best measure of the differences in distribution shapes seen above in Fig. 9 (see supplementary material). With a greater sample size from both sexes across the cemetery, this picture could change but it does show that there was a large portion of the population with ‘non-local’ signatures in both sexes. Given the diachronic changes above, perhaps marriage practices changed through time, or there were more local women and a gender imbalance during the seventh century in the cemetery, with males being buried elsewhere. This issue of a lack of male burials dated to later phases is a well-known problem in early medieval cemeteries, with a tendency to date ‘male’ grave goods such as weapons as significantly earlier than ‘female’ dress accessories which show more stylistic changes through time (Bayliss et al. 2013; Brownlee 2019).
Finglesham could therefore represent a largely immigrant population with incomers of both sexes, who may have practiced exogamy later in time with a mixture of ‘local’ and ‘non-local’ females and males present; however, a larger sample size from both sexes and better dates for male graves in particular would be needed to confirm this.
Do these sex-based differences in height and ∆18Odw-MAP values bear out in diet? Are these differences in height due to nutrition more so than genetics, given that many females had similar childhood origins to the males?
What Fig. 10 and Fig. 11 show is that there is an almost complete overlap in bone values between females and males, but this is not the case for dentine. Despite the high degree of overlap in bag plots between female and male bone δ13Ccoll and δ15Ncoll values, the inner bags are very different in shape. Diet in childhood, represented by dentine, is where the real differences lie with a high degree of overlap, but ~ 50% of males having less negative \(\delta\) 13Ccoll and slightly lower δ15Ncoll values than females. Males occupy a larger isotopic niche in childhood in the Finglesham subset. Therefore, the question is, are these differences due to dietary or physiological differences? Or are they so small that they may be biochemically ephemeral? The relatively small differences suggest it might be the latter and are consistent with offsets between the sexes found in modern Japanese individuals (Minagawa 1992).
Detecting deviants analysis
As outlined above DDC analysis looks for outliers on a cell-wise basis, meaning here that it flags variables for burials which stand out given their other contextual information (e.g. lower than expected dietary signatures given the number of grave goods they have or stature). Six individuals (graves 16, 106, 118, 133, 168 and 17) were removed by DDC due to having over 50% missing data, leaving 40 individuals suitable for DDC analysis. The results can be seen in Fig. 12.
Six individuals who stand out with three or more deviating cells are summarised in Table 2. There is a mixture of females and males, and there are no clear trends in what variables they deviate in or their contextual data. Four of the individuals are coffin burials or possible coffin burials, and all bar one of these individuals are aligned roughly W-E, however, as is clear in Fig. 2, most burials sampled are broadly on a W-E axis.
Grave 15 and grave 165 should be highlighted in particular, as they have the most deviating cells (four and five respectively) for the site. Grave 15 is flagged for number of grave goods (high), their δ13Ccoll values (low) and therefore also ∆13Cdentine-bone (high). Grave 165 was flagged for five variables (see above) and is a clear outlier for their community in terms of their N-S orientation but also their diet, especially in childhood, and the change between dentine and bone in terms of δ13Ccoll values. Both of these individuals, whilst not outliers in DDC for oxygen (due to the high proportion of migrants in the cemetery), have ∆18Odw-MAP values which suggest they are non-locals, so perhaps their deviance in diet is due to their foodways before coming to Kent. Using other methods, these individuals would not have been highlighted as particularly different from the rest of the group, so this certainly opens up avenues for further investigation.
It is interesting to note that the individuals who are outliers for oxygen (both δ18Ophosphate and ∆18Odw-MAP) in Fig. 12 stand out as higher than predicted for both values, and all are female, except grave 105. Their ∆18Odw-MAP values are all over + 1‰, highlighting that the majority of the cemetery’s population with negative values is seen by the algorithm as the ‘norm’.
When this is compared with using the \(\pm\) 2‰ criteria for ∆18Odw-MAP values, there are 19/40 individuals who fall outside the ‘local’ range for Finglesham. Conversely these are mostly male burials (14/19), and the one outlier above + 2‰ is the female grave 21B. Five of these burials are flagged by DDC as outliers for a variety of variables, but not necessarily for oxygen isotope values. There do appear to be some key underlying differences in enamel δ18Ophosphate and ∆18Odw-MAP values between female and male burials at Finglesham, and a tension between what is ‘normal’ for the cemetery in terms of mobility signatures versus the underlying ‘local’ isoscapes. The DDC analyses have supported the fact that there does not seem to be any particular link between some aspects of burial practice, mobility or diet within this community. Dietary outliers are rarely also mobility outliers, and neither are particularly linked to other non-isotopic variables.
Conclusions
From these analyses, it is clear that Finglesham is a mostly migrant population where change in diet from childhood to later in life, particularly in males, is visible. This suggests that these people spent quite some time in the region for their bone isotopic signatures to become more homogenised to reflect ‘Kentish’ or perhaps Finglesham specific resources and foodways. Those who stand out in terms of bone values may be more recent incomers to the area. The migration pattern at Finglesham supports this as they seem to be gendered, with more males being identified as ‘non-locals’. These dietary patterns and male mobility support a model of incoming male migrants and their acculturation once in Kent, at least in terms of foodways. This suggests local resources and dietary syncretism/acculturation win out here over imported childhood foodways.
Furthermore, the suggestion of freshwater fish consumption in the isotopic signatures at Finglesham aligns with zooarchaeological patterns seen at high status sites like Lyminge as they underwent Christianisation as per the laws of Wihtred of Kent (Knapp 2018; Thomas 2013; Thomas and Knox 2012; Whitelock 1994).Footnote 2
It has been demonstrated here that the community buried at Finglesham in Kent were from a diverse range of origins, and that migration to the area was likely continuous throughout the first millennium AD. Migrants could have come from areas of the Mediterranean up to northern parts of Scandinavia, with brewing and stewing also a potential factor for some individuals. This diversity in origins does not appear to correlate with any particular number or style of grave goods. The debated expressions and changes in ‘ethnic’ identities theoretically signalled by certain objects like brooches have far more complex cultural meanings and processes of construction behind them and are not closely associated with individuals with any particular isotopic signatures.
The cemetery at Finglesham provides new evidence for the ‘Adventus Saxonum’ and continuing migration from the Continent to Kent after the fifth and early sixth century AD. The isotopic data when combined with the other archaeological evidence from the cemetery demonstrate the cultural integration of these migrants into the community both in life and in death.
Data availability
Data for this paper are drawn from Leggett et al. 2021 (https://doi.org/10.1002/ecy.3349, also available at https://doi.org/10.17863/CAM.58286); all other additional data are available in the paper or as Supplementary Material.
Code availability
R scripts used to generate figures and statistical analyses for this paper are available in the supplementary material to this paper.
Notes
It is important to note here that these sources whilst medieval are not contemporary with the events they describe, the closest is Gildas (c. sixth century) and then Bede’s HE (Historia Ecclesiastica Gentis Anglorum – The Ecclesiastical History of the English People) which post-dates the migrations by c. 300 years (written c. 731 AD).
The laws of Wihtred (c. 695 AD) state the following in regard to Christian dietary prohibitions: “If anyone gives meat to his household in time of fasting, he is to redeem both freeman and slave with healsfang. If a slave eat it of his own accord [he is to pay] six shillings or be flogged.” (Whitelock, 1994).
References
Alt KW, Knipper C, Peters D et al (2014) Lombards on the Move – an integrative study of the migration period cemetery at Szólád Hungary. Plos ONE Bondioli L (ed) 9(11):e110793. https://doi.org/10.1371/journal.pone.0110793
Ambrose SH (1986) Stable carbon and nitrogen isotope analysis of human and animal diet in Africa. J Hum Evol 15(8):707–731. https://doi.org/10.1016/S0047-2484(86)80006-9
Ambrose SH (1991) Effects of diet, climate and physiology on nitrogen isotope abundances in terrestrial foodwebs. J Archaeol Sci 18(3):293–317. https://doi.org/10.1016/0305-4403(91)90067-Y
Ambrose SH, Norr L (1993) Experimental evidence for the relationship of the carbon isotope ratios of whole diet and dietary protein to those of bone collagen and carbonate. In: Lambert JB and Grupe G (eds) Prehistoric human bone. Berlin, Heidelberg: Springer Berlin Heidelberg, pp. 1–37. https://doi.org/10.1007/978-3-662-02894-0_1
Attali D, Baker C (2019) GgExtra: add marginal histograms to ‘Ggplot2’, and more ‘ggplot2’ enhancements. Available at: https://CRAN.R-project.org/package=ggExtra (accessed 4 June 2019).
Balasse M, Ambrose SH, Smith AB et al (2002) The seasonal mobility model for prehistoric herders in the south-western Cape of South Africa assessed by isotopic analysis of sheep tooth enamel. J Archaeol Sci 29(9):917–932. https://doi.org/10.1006/jasc.2001.0787
Bayliss A, Scull C, McCormac G, et al (2013) Anglo-Saxon graves and grave goods of the 6th and 7th centuries AD: a chronological framework. In: Hines J, Bayliss A (eds) The Society for Medieval Archaeology monograph 33. Society for Medieval Archaeology, London
Beaumont J, Gledhill A, Lee-Thorp J et al (2013) Childhood diet: a closer examination of the evidence from dental tissues using stable isotope analysis of incremental human dentine. Archaeometry 55(2):277–295
Beaumont J, Montgomery J, Buckberry J et al (2015) Infant mortality and isotopic complexity: New approaches to stress, maternal health, and weaning. Am J Phys Anthropol 157(3):441–457. https://doi.org/10.1002/ajpa.22736
Blair PH (2003) An Introduction to Anglo-Saxon England, 3rd edn. Cambridge University Press, Cambridge, UK
Bocherens H, Drucker D (2003) Trophic level isotopic enrichment of carbon and nitrogen in bone collagen: case studies from recent and ancient terrestrial ecosystems. Int J Osteoarchaeol 13(1–2):46–53. https://doi.org/10.1002/oa.662
Bond AL, Jardine TD, Hobson KA (2016) Multi-tissue stable-isotope analyses can identify dietary specialization. Methods Ecol Evol 7(12):1428–1437. https://doi.org/10.1111/2041-210X.12620
Bowen GJ (2019) The online isotopes in precipitation calculator, version 3.2. Available at: http://www.waterisotopes.org.
Bowen GJ, Revenaugh J (2003) Interpolating the isotopic composition of modern meteoric precipitation. Water Resour Res 39(10). https://doi.org/10.1029/2003WR002086
Brettell R, Montgomery J, Evans J (2012a) Brewing and stewing: the effect of culturally mediated behaviour on the oxygen isotope composition of ingested fluids and the implications for human provenance studies. J Anal at Spectrom 27(5):778. https://doi.org/10.1039/c2ja10335d
Brettell R, Evans J, Marzinzik S et al (2012b) ‘Impious Easterners’: can oxygen and strontium isotopes serve as indicators of provenance in early medieval European cemetery populations? Eur J Archaeol 15(1):117–145. https://doi.org/10.1179/1461957112Y.0000000001
British Geological Survey (n.d.) Geology of Britain viewer | British Geological Survey (BGS). Available at: http://mapapps.bgs.ac.uk/geologyofbritain/home.html? Accessed 27 June 2019
Brookes S, Harrington S (2010) The kingdom and people of Kent, AD 400–1066: their history and archaeology. The History Press Ltd, Stroud
Brown T, Brown K (2011) Biomolecular archaeology: an introduction. Wiley-Blackwell, Chichester
Brownlee EC (2019) Burial practices in transition: a study of the social, cultural and religious cohesion of early medieval Europe. University of Cambridge, Cambridge, PhD
Brownlee EC (2020) The dead and their possessions: the declining agency of the cadaver in early medieval Europe. Eur J Archaeol: 1–22. https://doi.org/10.1017/eaa.2020.3
Büntgen U, Myglan VS, Ljungqvist FC et al (2016) Cooling and societal change during the Late Antique Little Ice Age from 536 to around 660 AD. Nat Geosci 9(3):231–236. https://doi.org/10.1038/ngeo2652
Büntgen U, Myglan VS, Ljungqvist FC et al (2017) Reply to ‘Limited Late Antique cooling.’ Nat Geosci 10(4):243–243. https://doi.org/10.1038/ngeo2927
Calin-Jageman RJ, Cumming G (2019) The new statistics for better science: ask how much, how uncertain, and what else is known. Am Stat 73(sup1):271–280. https://doi.org/10.1080/00031305.2018.1518266
Chadwick Hawkes S, Grainger G (2006) The Anglo-Saxon cemetery at Finglesham, Kent (ed. B Brugmann). Oxford University School of Archaeology Monograph 64. Oxford: School of Archaeology, University of Oxford.
Chenery CA, Pashley V, Lamb AL et al (2012) The oxygen isotope relationship between the phosphate and structural carbonate fractions of human bioapatite: Relationship between phosphate and structural carbonate δ18O in human enamel. Rapid Commun Mass Spectrom 26(3):309–319. https://doi.org/10.1002/rcm.5331
Clementz MT, Koch PL (2001) Differentiating aquatic mammal habitat and foraging ecology with stable isotopes in tooth enamel. Oecologia 129(3):461–472. https://doi.org/10.1007/s004420100745
Clementz MT, Koch PL, Beck CA (2007) Diet induced differences in carbon isotope fractionation between sirenians and terrestrial ungulates. Mar Biol 151(5):1773–1784. https://doi.org/10.1007/s00227-007-0616-1
Clementz MT, Fox-Dobbs K, Wheatley PV et al (2009) Revisiting old bones: coupled carbon isotope analysis of bioapatite and collagen as an ecological and palaeoecological tool. Geol J 44(5):605–620. https://doi.org/10.1002/gj.1173
Collaboration OS (2015) Estimating the reproducibility of psychological science. Science 349(6251). https://doi.org/10.1126/science.aac4716
Crawford S (2018) 1. Childhood and adolescence: interdisciplinary approaches to the archaeological and documentary evidence. In: Irvine S and Rudolf W (eds) Childhood & adolescence in Anglo-Saxon literary culture. Toronto: University of Toronto Press, pp. 15–31. https://doi.org/10.3138/9781487514433-004
Di Cosmo N, Oppenheimer C, Büntgen U (2017) Interplay of environmental and socio-political factors in the downfall of the Eastern Türk Empire in 630 CE. Clim Change 145(3–4):383–395. https://doi.org/10.1007/s10584-017-2111-0
Evans JA, Chenery CA, Montgomery J (2012) A summary of strontium and oxygen isotope variation in archaeological human tooth enamel excavated from Britain. J Anal at Spectrom 27(5):754–764. https://doi.org/10.1039/c2ja10362a
Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 40(1):503–537. https://doi.org/10.1146/annurev.pp.40.060189.002443
Fei J, Zhou J and Hou Y (2007) Circa a.d. 626 volcanic eruption, climatic cooling, and the collapse of the Eastern Turkic Empire. Climatic Change 81(3–4): 469–475. https://doi.org/10.1007/s10584-006-9199-y
France CAM, Owsley DW (2015) Stable carbon and oxygen isotope spacing between bone and tooth collagen and hydroxyapatite in human archaeological remains: stable carbon and oxygen isotope spacing in humans. Int J Osteoarchaeol 25(3):299–312. https://doi.org/10.1002/oa.2300
Fuller BT, Fuller JL, Sage NE et al (2005) Nitrogen balance and δ15N: why you’re not what you eat during nutritional stress. Rapid Commun Mass Spectrom 19(18):2497–2506. https://doi.org/10.1002/rcm.2090
Fuller BT, Molleson TI, Harris DA et al (2006) Isotopic evidence for breastfeeding and possible adult dietary differences from late/sub-Roman Britain. Am J Phys Anthropol 129(1):45–54. https://doi.org/10.1002/ajpa.20244
Gannes LZ, del Rio CM, Koch P (1998) Natural abundance variations in stable isotopes and their potential uses in animal physiological ecology. Comp Biochem Physiol a: Mol Integr Physiol 119(3):725–737. https://doi.org/10.1016/S1095-6433(98)01016-2
Ganzarolli G, Alexander M, Chavarria Arnau A et al (2018) Direct evidence from lipid residue analysis for the routine consumption of millet in Early Medieval Italy. J Archaeol Sci 96:124–130. https://doi.org/10.1016/j.jas.2018.06.007
Gildas (2010) De Excidio Britanniae; Or, the Ruin of Britain (tran. HA Williams). Milton Keynes: Dodo Press
Gregoricka LA, Sheridan SG, Schirtzinger M (2017) Reconstructing life histories using multi-tissue isotope analysis of commingled remains from St Stephen’s Monastery in Jerusalem: limitations and potential: analysis of remains from St Stephen’s Monastery in Jerusalem. Archaeometry 59(1):148–163. https://doi.org/10.1111/arcm.12227
Groves SE, Roberts CA, Lucy S et al (2013) Mobility histories of 7th-9th century AD people buried at early medieval Bamburgh, Northumberland, England: Mobility Histories At Bamburgh England. Am J Phys Anthropol 151(3):462–476. https://doi.org/10.1002/ajpa.22290
Hagen A (2006) Anglo-Saxon food & drink. Anglo-Saxon Books, Ely
Hakenbeck S (2013) Potentials and limitations of isotope analysis in Early Medieval archaeology. Post-Classical Archaeologies 3:109–125
Hakenbeck SE, Evans J, Chapman H et al (2017) Practising pastoralism in an agricultural environment: An isotopic analysis of the impact of the Hunnic incursions on Pannonian populations. PLOS ONE Caramelli D (ed) 12(3):e0173079. https://doi.org/10.1371/journal.pone.0173079
Hamerow H (1997) Migration theory and the Anglo-Saxon ‘identity crisis’. In: Chapman J and Hamerow H (eds) Migrations and invasions in archaeological explanation. BAR International Series 664. Oxford: BAR Publishing, pp. 33–44
Hamerow H, Bogaard A, Charles M, et al (2020) An integrated bioarchaeological approach to the medieval ‘agricultural revolution’: a case study from Stafford, England, c.ad 800–1200. Eur J Archaeol 1–25. https://doi.org/10.1017/eaa.2020.6
Härke H (2003) Population replacement or acculturation? An archaeological perspective on population and migration in post-Roman Britain. In: Tristram HLC (ed.) The Celtic Englishes III (Anglistische Forschungen, 324). Heidelberg, pp. 13–28
Härke H (2004) The debate on migration and identity in Europe. Antiquity 78(300):453–456
Härke H (2011) Anglo-Saxon immigration and ethnogenesis. Mediev Archaeol 55(1):1–28. https://doi.org/10.1179/174581711X13103897378311
Haydock H, Clarke L, Craig-Atkins E et al (2013) Weaning at Anglo-Saxon Raunds: Implications for changing breastfeeding practice in britain over two millennia: Weaning at Anglo-Saxon Raunds. Am J Phys Anthropol 151(4):604–612. https://doi.org/10.1002/ajpa.22316
Hedges REM (2003) On bone collagen — apatite-carbonate isotopic relationships. Int J Osteoarchaeol 13(1–2):66–79. https://doi.org/10.1002/oa.660
Hedges REM, Reynard LM (2007) Nitrogen isotopes and the trophic level of humans in archaeology. J Archaeol Sci 34(8):1240–1251. https://doi.org/10.1016/j.jas.2006.10.015
Hedges REM, Clement JG, Thomas CDL et al (2007) Collagen turnover in the adult femoral mid-shaft: Modeled from anthropogenic radiocarbon tracer measurements. Am J Phys Anthropol 133(2):808–816. https://doi.org/10.1002/ajpa.20598
Helama S, Jones PD and Briffa KR (2017) Limited Late Antique cooling. Nature Geoscience 10(4). 4. Nature Publishing Group: 242–243. DOI: https://doi.org/10.1038/ngeo2926.
Hemer KA, Evans JA, Chenery CA et al (2013) Evidence of early medieval trade and migration between Wales and the Mediterranean Sea region. J Archaeol Sci 40(5):2352–2359. https://doi.org/10.1016/j.jas.2013.01.014
Hill D, Cowie R (eds) (2001) Wics: the early mediaeval trading centres of northern Europe. Sheffield Archaeological Monographs. Continuum International Publishing Group Ltd., Sheffield, UK
Hills C (2015) The Anglo-Saxon migration: an archaeological case study of disruption. In: Baker BJ and Tsuda T (eds) Migration and disruptions: toward a unifying theory of ancient and contemporary migrations. University Press of Florida, pp. 33–51
Hills CM (2013a) Anglo-Saxon migration: historical fact or mythical fiction? - Guy Halsall. Worlds of Arthur: facts and fictions of the Dark Ages. xx+384 pages, 20 b&w illustrations, 15 maps. 2013. Oxford: Oxford University Press; 978–0–1996–5817–6 hardback £ 20. - Stephen Yeates. Myth and history. Ethnicity and politics in the first millennium British Isles. xi+348 pages, 104 b&w illustrations. 2012. Oxford & Oakville (CT): Oxbow; 978–1–84217–478–4 paperback £ 29.95. Antiquity 87(338). Cambridge University Press: 1220–1222. https://doi.org/10.1017/S0003598X00050006
Hills CM (2013b) Anglo-Saxon migrations. In: The Encyclopedia of Global Human Migration. American Cancer Society. https://doi.org/10.1002/9781444351071.wbeghm029
Hoefs J (2018) Stable Isotope Geochemistry. Eighth edition. Springer textbooks in earth sciences, geography and environment. Cham, Switzerland: Springer. Available at: https://cam.ldls.org.uk/vdc_100072715289.0x000001 (accessed 2 January 2020)
Howland MR, Corr LT, Young SMM et al (2003) Expression of the dietary isotope signal in the compound-specific δ13C values of pig bone lipids and amino acids. Int J Osteoarchaeol 13(1–2):54–65. https://doi.org/10.1002/oa.658
Hughes SS, Millard AR, Lucy SJ et al (2014) Anglo-Saxon origins investigated by isotopic analysis of burials from Berinsfield, Oxfordshire, UK. J Archaeol Sci 42:81–92. https://doi.org/10.1016/j.jas.2013.10.025
Hughes SS, Millard AR, Chenery CA et al (2018) Isotopic analysis of burials from the early Anglo-Saxon cemetery at Eastbourne, Sussex, U.K. J Archaeol Sci Rep 19:513–525. https://doi.org/10.1016/j.jasrep.2018.03.004
Iacumin P, Bocherens H, Mariotti A et al (1996) Oxygen isotope analyses of co-existing carbonate and phosphate in biogenic apatite: a way to monitor diagenetic alteration of bone phosphate? Earth Planet Sci Lett 142(1):1–6. https://doi.org/10.1016/0012-821X(96)00093-3
Jay M (2005) STABLE ISOTOPE EVIDENCE FOR BRITISH IRON AGE DIET: Inter- and intra-site variation in carbon and nitrogen from bone collagen at Wetwang in East Yorkshire and sites in East Lothian, Hampshire and Cornwall. PhD. University of Bradford, Bradford, UK.
Keller M, Spyrou MA, Scheib CL, et al. (2019) Ancient Yersinia pestis genomes from across Western Europe reveal early diversification during the First Pandemic (541–750). Proc Nat Acad Sci: 201820447. https://doi.org/10.1073/pnas.1820447116
Kellner CM, Schoeninger MJ (2007) A simple carbon isotope model for reconstructing prehistoric human diet. Am J Phys Anthropol 133(4):1112–1127. https://doi.org/10.1002/ajpa.20618
Knapp Z (2018) The zooarchaeology of the Anglo-Saxon Christian conversion: Lyminge, a case study. University of Reading, Reading, PhD
Kruschke J (2014) Doing Bayesian data analysis: a tutorial with R, JAGS, and Stan, 2nd edn. Academic Press, Boston
Kruschke JK (2013) Bayesian estimation supersedes the t test. J Exp Psychol Gen 142(2):573–603. https://doi.org/10.1037/a0029146
Kruschke JK, Liddell TM (2018) The Bayesian new statistics: hypothesis testing, estimation, meta-analysis, and power analysis from a Bayesian perspective. Psychon Bull Rev 25(1):178–206. https://doi.org/10.3758/s13423-016-1221-4
Krzewińska M, Kjellström A, Günther T et al (2018) Genomic and strontium isotope variation reveal immigration patterns in a Viking Age town. Curr Biol 28(17):2730-2738.e10. https://doi.org/10.1016/j.cub.2018.06.053
Kusaka S (2019) Stable isotope analysis of human bone hydroxyapatite and collagen for the reconstruction of dietary patterns of hunter-gatherers from Jomon populations. Int J Osteoarchaeol 29(1):36–47. https://doi.org/10.1002/oa.2711
Kusaka S, Uno KT, Nakano T et al (2015) Carbon isotope ratios of human tooth enamel record the evidence of terrestrial resource consumption during the Jomon period Japan. Am J Phys Anthropol 158(2):300–311. https://doi.org/10.1002/ajpa.22775
Lamb AL, Melikian M, Ives R et al (2012) Multi-isotope analysis of the population of the lost medieval village of Auldhame, East Lothian Scotland. J Anal Atom Spectrom 27(5):765. https://doi.org/10.1039/c2ja10363j
Lamb AL, Evans JE, Buckley R et al (2014) Multi-isotope analysis demonstrates significant lifestyle changes in King Richard III. J Archaeol Sci 50:559–565. https://doi.org/10.1016/j.jas.2014.06.021
Lavine M (2019) Frequentist, Bayes, or Other? Am Stat 73(sup1):312–318. https://doi.org/10.1080/00031305.2018.1459317
Lee-Thorp JA, Sealy JC, van der Merwe NJ (1989) Stable carbon isotope ratio differences between bone collagen and bone apatite, and their relationship to diet. J Archaeol Sci 16(6):585–599. https://doi.org/10.1016/0305-4403(89)90024-1
Leggett S (2017) ‘Monarchies and monasteries’ - christianisation and centralisation along Anglo-Saxon waterways. University of Sydney, Sydney, MPhil (research)
Leggett S, Rose A, Praet E et al (2021) Multi-tissue and multi-isotope (δ13C, δ15N, δ18O and 87/86Sr) data for early medieval human and animal palaeoecology. Ecology 102(6):e03349. https://doi.org/10.1002/ecy.3349
Lightfoot E, O’Connell TC (2016) On the use of biomineral oxygen isotope data to identify human migrants in the archaeological record: intra-sample variation, statistical methods and geographical considerations. PLoS ONE 11(4):e0153850. https://doi.org/10.1371/journal.pone.0153850
Lin GP, Rau YH, Chen YF et al (2003) Measurements of δD and δ18O Stable Isotope Ratios in Milk. J Food Sci 68(7):2192–2195. https://doi.org/10.1111/j.1365-2621.2003.tb05745.x
Longin R (1971) New method of collagen extraction for radiocarbon dating. Nature 230(5291):241–242. https://doi.org/10.1038/230241a0
Longinelli A (1984) Oxygen isotopes in mammal bone phosphate: a new tool for paleohydrological and paleoclimatological research? Geochim Cosmochim Acta 48(2):385–390. https://doi.org/10.1016/0016-7037(84)90259-X
Loveluck CP, McCormick M, Spaulding NE et al (2018) Alpine ice-core evidence for the transformation of the European monetary system, AD 640–670. Antiquity 92(366):1571–1585. https://doi.org/10.15184/aqy.2018.110
Lucy S, Newman R, Dodwell N et al (2009) The burial of a princess? The later seventh-century cemetery at Westfield Farm Ely. Antiquar J 89:81. https://doi.org/10.1017/S0003581509990102
Luterbacher J, Werner JP, Smerdon JE et al (2016) European summer temperatures since Roman times. Environ Res Lett 11(2):024001. https://doi.org/10.1088/1748-9326/11/2/024001
Makarewicz CA and Sealy J (2015) Dietary reconstruction, mobility, and the analysis of ancient skeletal tissues: Expanding the prospects of stable isotope research in archaeology. Journal of Archaeological Science 56. Scoping the Future of Archaeological Science: Papers in Honour of Richard Klein: 146–158. https://doi.org/10.1016/j.jas.2015.02.035
Marwick B (2018) Basic bagplot geom for ggplot2. Available at: https://gist.github.com/benmarwick/00772ccea2dd0b0f1745 (accessed 4 August 2018).
McElreath R (2018) Statistical rethinking: A Bayesian course with examples in R and Stan. 1st ed. Chapman and Hall/CRC. https://doi.org/10.1201/9781315372495
Michelet FL (2017) Adventus Saxonum. In: The Encyclopedia of Medieval Literature in Britain. American Cancer Society, pp. 1–5. https://doi.org/10.1002/9781118396957.wbemlb298
Minagawa M (1992) Reconstruction of human diet from σ13C and σ15N in contemporary Japanese hair: a stochastic method for estimating multi-source contribution by double isotopic tracers. Appl Geochem 7(2):145–158. https://doi.org/10.1016/0883-2927(92)90033-Y
Mordechai L, Eisenberg M, Newfield TP et al (2019) The Justinianic Plague: an inconsequential pandemic? Proc Natl Acad Sci 116(51):25546–25554. https://doi.org/10.1073/pnas.1903797116
O’Connell TC, Kneale CJ, Tasevska N et al (2012) The diet-body offset in human nitrogen isotopic values: A controlled dietary study. Am J Phys Anthropol 149(3):426–434. https://doi.org/10.1002/ajpa.22140
O’Leary MH (1988) Carbon isotopes in photosynthesis. Bioscience 38(5):328–336. https://doi.org/10.2307/1310735
Passey BH, Robinson TF, Ayliffe LK et al (2005) Carbon isotope fractionation between diet, breath CO2, and bioapatite in different mammals. J Archaeol Sci 32(10):1459–1470. https://doi.org/10.1016/j.jas.2005.03.015
Pattison JE (2008) Is it necessary to assume an apartheid-like social structure in Early Anglo-Saxon England? Proc Royal Soc B: Biol Sci 275(1650):2423–2429. https://doi.org/10.1098/rspb.2008.0352
Pederzani S, Britton K (2019) Oxygen isotopes in bioarchaeology: Principles and applications, challenges and opportunities. Earth Sci Rev 188:77–107. https://doi.org/10.1016/j.earscirev.2018.11.005
Pestell T (2011) Markets, emporia, wics, and ‘productive’ sites: pre-Viking trade centres in Anglo-Saxon England. In: Hamerow H, Hinton DA, and Crawford S (eds) The Oxford handbook of Anglo-Saxon archaeology. Oxford, UK: Oxford University Press
Pestle WJ, Crowley BE and Weirauch MT (2014) Quantifying Inter-Laboratory Variability in Stable Isotope Analysis of Ancient Skeletal Remains. PLOS ONE 9(7). Public Library of Science: e102844. https://doi.org/10.1371/journal.pone.0102844
Price TD, Burton JH (2012) An introduction to archaeological chemistry. Springer, New York
Privat KL, O’Connell TC, Richards MP (2002) Stable isotope analysis of human and faunal remains from the Anglo-Saxon cemetery at Berinsfield, Oxfordshire: dietary and social implications. J Archaeol Sci 29(7):779–790. https://doi.org/10.1006/jasc.2001.0785
QGIS (2020) Python. Available at: http://qgis.org.
R Development Core Team (2021) R: a language and environment for statistical computing. R. Available at: http://www.r-project.org/
Raymaekers J, Rousseeuw P, Bossche WV den, et al. (2020) CellWise: analyzing data with cellwise outliers. Available at: https://CRAN.R-project.org/package=cellWise.
Richards MP, Hedges REM (1999) Stable isotope evidence for similarities in the types of marine foods used by Late Mesolithic humans at sites along the Atlantic coast of Europe. J Archaeol Sci 26(6):717–722. https://doi.org/10.1006/jasc.1998.0387
Richards MP, Mays S, Fuller BT (2002) Stable carbon and nitrogen isotope values of bone and teeth reflect weaning age at the Medieval Wharram Percy site, Yorkshire UK. Am J Phys Anthropol 119(3):205–210. https://doi.org/10.1002/ajpa.10124
Richardson A (2005) The Anglo-Saxon Cemeteries of Kent. BAR British Series 391. Oxford: British Archaeological Reports.
Rousseeuw PJ, Bossche WVD (2018) Detecting deviating data cells. Technometrics 60(2):135–145. https://doi.org/10.1080/00401706.2017.1340909
Rousseeuw PJ, Ruts I, Tukey JW (1999) The bagplot: a bivariate boxplot. The American Statistician 53(4). Taylor & Francis: 382–387. https://doi.org/10.1080/00031305.1999.10474494
Rozanski K, Araguás-Araguás L, Gonfiantini R (1992) Relation between long-term trends of oxygen-18 isotope composition of precipitation and climate. Science 258(5084):981–985. https://doi.org/10.1126/science.258.5084.981
Rozanski K, Araguás-Araguás L, Gonfiantini R (2013) Isotopic patterns in modern global precipitation. In: Swart PK, Lohmann KC, Mckenzie J, et al. (eds) Geophysical monograph series. Washington, D. C.: American Geophysical Union, pp. 1–36. https://doi.org/10.1029/GM078p0001
RStudio Team (2021) RStudio: integrated development environment for R. R. Boston, MA: RStudio, Inc. Available at: http://www.rstudio.com/.
Ryan SE, Reynard LM, Crowley QG et al (2018) Early medieval reliance on the land and the local: An integrated multi-isotope study (87Sr/86Sr, δ18O, δ13C, δ15N) of diet and migration in Co., Meath Ireland. J Archaeol Sci 98:59–71. https://doi.org/10.1016/j.jas.2018.08.002
Scheid RC (2007) Woelfel’s dental anatomy: its relevance to dentistry, 7th edn. Lippincott Williams & Wilkins, Baltimore
Sealy J, Armstrong R, Schrire C (1995) Beyond lifetime averages: tracing life histories through isotopic analysis of different calcified tissues from archaeological human skeletons. Antiquity 69(263):290–300. https://doi.org/10.1017/S0003598X00064693
Smith K, Rennie M (2007) New approaches and recent results concerning human-tissue collagen synthesis. Curr Opin Clin Nutr Metab Care 10(5):582–590. https://doi.org/10.1097/MCO.0b013e328285d858
Sorensen P (1999) A reassessment of the Jutish nature of Kent, southern Hampshire and the Isle of Wight. Ph.D. University of Oxford. Available at: https://ethos.bl.uk/OrderDetails.do?uin=uk.bl.ethos.312632
Soulat J (2013) Study of Merovingian-type grave-goods in Kent: a first approach. In: Ludowici B (ed.) Individual or Individuality? Approaches towards an Archaeology of Personhood in the First Millennium AD. Neue Studien zur Sachsenforschung 4. Hannover: Niedersächsusches Landesmuseum Hannover, pp. 205–216
Soulat J (2018) Le mobilier funéraire de type franc et mérovingien dans le Kent et sa périphérie. Europe Médiévale 13. Dremil-Lafage: Editions Mergoil.
Stenton FM (1971) Anglo-Saxon England. 3rd ed. The Oxford history of England 2. Oxford: Clarendon Press
Stoodley N (2011) Childhood to old age. In: Hinton DA, Crawford S, and Hamerow H (eds) The Oxford handbook of Anglo-Saxon archaeology. Oxford Handbooks. Oxford: Oxford University Press. https://doi.org/10.1093/oxfordhb/9780199212149.013.0033
The Venerable Bede (2009) Historia Ecclesiastica Gentis Anglorum (eds J McClure and R Collins; tran. B Colgrave). Oxford: Oxford University Press
Thomas G (2013) Life before the Minster: the social dynamics of monastic foundation at Anglo-Saxon Lyminge, Kent. Antiqu J 93:109–145. https://doi.org/10.1017/S0003581513000206
Thomas G and Knox A (2012) A window on Christianisation: transformation at Anglo-Saxon Lyminge, Kent, England. Antiquity Bulletin. Available at: http://www.antiquity.ac.uk/projgall/thomas334/ (accessed 6 June 2019)
Thomas MG, Stumpf MPH, Härke H (2006) Evidence for an apartheid-like social structure in early Anglo-Saxon England. Proc Royal Soc B: Biol Sci 273(1601):2651–2657. https://doi.org/10.1098/rspb.2006.3627
Thomas MG, Stumpf MPH, Härke H (2008) Integration versus apartheid in post-Roman Britain: a response to Pattison. Proc Royal Soc B: Biol Sci 275(1650):2419–2421. https://doi.org/10.1098/rspb.2008.0677
Tieszen LL, Fagre T (1993) Effect of diet quality and composition on the isotopic composition of respiratory CO2, bone collagen, bioapatite, and soft tissues. In: Lambert JB and Grupe G (eds) Prehistoric human bone: archaeology at the molecular level. Berlin/Heidelberg: Springer, pp. 121–155. https://doi.org/10.1007/978-3-662-02894-0_5
Tukey JW (1977) Exploratory data analysis. Addison-Wesley series in behavioural science : quantitative methods. Addison-Wesley, Reading, Mass.
Unknown (2008) The Anglo-Saxon chronicle. Available at: http://avalon.law.yale.edu/subject_menus/angsax.asp.
van Klinken GJ (1999) Bone collagen quality indicators for palaeodietary and radiocarbon measurements. J Archaeol Sci 26(6):687–695. https://doi.org/10.1006/jasc.1998.0385
Wasserstein RL, Lazar NA (2016) The ASA statement on p-values: context, process, and purpose. Am Stat 70(2):129–133. https://doi.org/10.1080/00031305.2016.1154108
Wasserstein RL, Schirm AL and Lazar NA (2019) Moving to a world beyond “p < 0.05”. The American Statistician 73(sup1): 1–19. https://doi.org/10.1080/00031305.2019.1583913
Webb EC, Lewis J, Shain A, et al. (2017) The influence of varying proportions of terrestrial and marine dietary protein on the stable carbon-isotope compositions of pig tissues from a controlled feeding experiment. STAR: Sci Technol Archaeol Res 3(1): 28–44. https://doi.org/10.1080/20548923.2016.1275477
Welch M (2011) The Mid Saxon ‘Final Phase’. In: Hamerow H, Hinton DA, and Crawford S (eds) The Oxford Handbook of Anglo-Saxon Archaeology. Oxford, UK: Oxford University Press
White CD, Spence MW, Longstaffe FJ et al (2002) Geographic Identities of the Sacrificial Victims from the Feathered Serpent Pyramid, Teotihuacan: Implications for the Nature of State Power. Latin American Antiquity 13(2). Cambridge University Press: 217–236. https://doi.org/10.2307/971915
White CD, Spence MW, Longstaffe FJ et al (2004) Demography and ethnic continuity in the Tlailotlacan enclave of Teotihuacan: the evidence from stable oxygen isotopes. Journal of Anthropological Archaeology 23(4):385–403. https://doi.org/10.1016/j.jaa.2004.08.002
Whitelock D (1994) The laws of Wihtred, King of Kent (695). In: English Historical Documents Volume I, c.500–1042. English Historical Documents. London; New York: Routledge. Available at: http://search.ebscohost.com/login.aspx?direct=true&scope=site&db=nlebk&db=nlabk&AN=85138.
Wickham H (2019) Ggplot2: elegant graphics for data analysis. 3rd Edition. Available at: https://ggplot2-book.org/.
Wickham H and Stryjewski L (2012) 40 years of boxplots. Available at: http://vita.had.co.nz/papers/boxplots.html.
Wilke CO (2020) Ggridges: Ridgeline Plots in ‘Ggplot2’. R. Available at: https://CRAN.R-project.org/package=ggridges.
Zhu M, Sealy J (2019) Multi-tissue stable carbon and nitrogen isotope models for dietary reconstruction: Evaluation using a southern African farming population. Am J Phys Anthropol 168(1):145–153. https://doi.org/10.1002/ajpa.23731
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1(1):3–14. https://doi.org/10.1111/j.2041-210X.2009.00001.x
Acknowledgements
I would like to thank my funders for supporting my doctoral research. I also thank my supervisors Susanne Hakenbeck and Tamsin O’Connell for reading and commenting on the thesis chapter this article originates from and their advice over the years, and to my examiners Helena Hamerow and James Barrett for their thoughtful comments and suggestions. Many thanks to Trish Biers and colleagues at the Duckworth Laboratory for giving permission for sampling and analysis of burials from Finglesham, and their support of my work. Thanks also to Ket Smithson for helping me to CT scan all of the samples before analysis. I also gratefully acknowledge the help of Catherine Kneale and James Rolfe for their technical assistance and expertise with running the mass spectrometers. Thanks must also go to Emma Brownlee and Erin Sebo for reading earlier versions of this article, and the Graduate Early Medieval Seminar group at Cambridge for the opportunity to workshop my work on Kent. And a huge thank you to all members of the Dorothy Garrod lab past and present for all of their advice and assistance throughout my time at Cambridge.
Funding
This work was supported by the Cambridge Trust and Newnham College through an international studentship (App No: 10386281) and a Cambridge Philosophical Society studentship.
Author information
Authors and Affiliations
Contributions
Not applicable, single author.
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Leggett, S. Migration and cultural integration in the early medieval cemetery of Finglesham, Kent, through stable isotopes. Archaeol Anthropol Sci 13, 171 (2021). https://doi.org/10.1007/s12520-021-01429-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12520-021-01429-7