Abstract
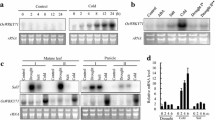
A full-length cDNA corresponding to ddOs319, previously isolated as a cold-responsive gene in the flowers by mRNA differential display (Plant Cell Rep 26:1097–1110, 2007), was obtained from the cold-treated flowers by reverse transcription and nested PCR. The cDNA encodes a putative class III peroxidase of 335 amino acids with 77–98% identity with rice peroxidases and named OsPOX1. To understand the regulation of OsPOX1 expression, a 1.8 kb promoter region of OsPOX1 was isolated and fused to β-glucuronidase (GUS) reporter gene. Transgenic rice plants expressing P OsPOX1 -GUS showed minimal GUS activity in both shoots and roots at the vegetative stage. In the flowers at early young microspore stage, GUS activity was detected in the veins and anthers. Interestingly, at the later vacuolated pollen stage, the GUS activity was highly induced by cold stress, suggesting that OsPOX1 is a flower-preferential cold-responsive gene in rice.





Similar content being viewed by others
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–411
An G, Ebert PR, Mitra A, Ha SB (1988) In: Gelvin SB, Schilperoort RA (eds) Binary vectors; in Plant Molecular Biology Manual. Kluwer Academic Publishers, Dordrecht, pp A3/1–A3/19
Baker SS, Wilhelm KS, Thomashow MF (1994) The 5′-region of Arabidopsis thaliana cor15a has cis-acting elements that confer cold-, drought- and ABA-regulated gene expression. Plant Mol Biol 24:1701–1713
Dolferus R, Jacobs M, Peacock WJ, Dennis ES (1994) Differential interactions of promoter elements in stress responses of the Arabidopsis Adh gene. Plant Physiol 105:1075–1087
Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6:271–282
Hiraga S, Sasaki K, Ito H, Ohashi Y, Matsui H (2001) A large family of class III plant peroxidases. Plant Cell Physiol 42:462–468
Hoshikawa K (1989) The growing rice plant: an anatomical monograph (1st ed). Nobunkyo, Minato-ku, Tokyo
Imin N, Kerim T, Weinman JJ, Rolfe BG (2006) Low temperature treatment at the young microspore stage induces protein changes in rice anthers. Mol Cell Proteom 5:1274–1292
Ito H, Hiraga S, Tsugawa H, Matsui H, Honma M, Otsuki Y, Murakami T, Ohashi Y (2000) Xylem-specific expression of wound-inducible rice peroxidase genes in transgenic plants. Plant Sci 155:85–100
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Kasuga M, Liu Q, Miura S, Yamaguchi-Shinozaki K, Shinozaki K (1999) Improving plant drought, salt and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat Biotechnol 17:287–291
Kim SH, Kim JY, Kim SJ, An K, An G, Kim SR (2007) Isolation of cold stress-responsive genes in the reproductive organs, and characterization of the OsLti6b gene from rice (Oryza sativa L.). Plant Cell Rep 26:1097–1110
KimYH YKS, Kim CY, Ryu SH, Song WK, Kwon SY, Lee HS, Bang JW, Kwak SS (2008) Molecular cloning of peroxidase cDNAs from dehydration-treated fibrous roots of sweet potato and their differential expression in response to stress. J Biochem Mol Biol 41:259–265
Koh S, Lee S-C, Kim M-K, Koh JH, Lee S, An G, Choe S, Kim S-R (2007) T-DNA tagged knockout mutation of rice OsGSK1, an orthologue of Arabidopsis BIN2, with enhanced tolerance to various abiotic stresses. Plant Mol Biol 65:453–466
Lee S, Jeon JS, Jung KH, An G (1999) Binary vectors for efficient transformation of rice. J Plant Biol 42:310–316
Lee SC, Huh KW, An K, An G, Kim SR (2004) Ectopic expression of a cold-inducible transcription factor, CBF1/DREB1b, in transgenic rice (Oryza sativa L.). Mol Cells 18:107–114
Llorente F, Lopez-Cobollo RM, Catala R, Martinez-Zapater JM, Salinas J (2002) A novel cold-inducible gene from Arabidopsis, RCI3, encodes a peroxidase that constitutes a component for stress tolerance. Plant J 32:13–24
Martin T, Wohner R, Hummel S, Willmitzer L, Frommer WB (1991) In: Gallagher SR (ed) The GUS reporter system as a tool to study plant gene expression; in GUS protocols—using the GUS gene as a reporter of gene expression. Academic, New York
Nicholas KB, Nicholas HBJ, Deerfield DW II (1997) GeneDoc: analysis and visualization of genetic variation. EMBNEW NEWS 4:14
Passardi F, Longet D, Penel C, Dunand C (2004) The class III peroxidase multigenic family in rice and its evolution in land plants. Phytochemistry 65:1879–1893
Saad RB, Romdhan WB, Zouari N, Azaza J, Mieulet D, Verdeil JL, Guiderdoni E, Hassairi A (2010) Promoter of the AlSAP gene from the halophyte grass Aeluropus littoralis directs developmental-regulated, stress-inducible, and organ-specific gene expression in transgenic tobacco. Transgenic Res. doi:10.1007/s11248-010-9474-6
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain terminating inhibitors. Proc Natl Acad Sci U S A 74:5463–5467
Sasaki K, Hiraga S, Ito H, Seo S, Matsui H, Ohashi Y (2002) A wound-inducible tobacco peroxidase gene expresses preferentially in the vascular system. Plant Cell Physiol 43:108–111
Sasaki K, Iwai T, Hiraga S, Kuroda K, Seo S, Mitsuhara I, Miyasaka A, Iwano M, Ito H, Matsui H, Ohashi Y (2004) Ten rice peroxidases redundantly respond to multiple stresses including infection with rice blast fungus. Plant Cell Physiol 45:1442–1452
Satake T, Hayase H (1970) Male sterility caused by cooling treatment at the young microspore stage in rice plants. V. Estimation of pollen developmental stage to coolness. Proc Crop Sci Soc Jpn 39:468–473
Sato Y, Masuta Y, Saito K, Murayama S, Ozawa K (2011) Enhanced chilling tolerance at the booting stage in rice by transgenic overexpression of the ascorbate peroxidase gene, OsAPXa. Plant Cell Rep 30:399–406
Thomashow MF (1999) Plant cold acclimation, freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol Plant Mol Biol 50:571–599
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The Clustal X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucl Acids Res 25:4876–4882
Toenniessen GH (1991) Potentially useful genes for rice genetic engineering. In: Khush GS, Toenniessen GH (eds) Rice Biotechnology. International Rice Research Institute and Wallingford (United Kingdom), CAB International, Manila
Tokunaga N, Kaneta T, Sato S, Sato Y (2009) Analysis of expression profiles of three peroxidase genes associated with lignification in Arabidopsis thaliana. Physiol Plant 136:237–249
Valerio L, De Meyer M, Penel C, Dunand C (2004) Expression analysis of the Arabidopsis peroxidase multigenic family. Phytochemistry 65:1331–1342
Welinder KG (1992) Superfamily of plant, fungal and bacterial peroxidases. Curr Opin Struct Biol 2:388–393
Weterings K, Schrauwen J, Wullems G, Twell D (1995) Functional dissection of the promoter of the pollen-specific gene NTP303 reveals a novel pollen-specific, and conserved cis-regulatory element. Plant J 8:55–63
Yamaguchi T, Nakayama K, Hayashi T, Yazaki J, Kishimoto N, Kikuchi S, Koike S (2004) cDNA microarray analysis of rice anther genes under chilling stress at the microsporogenesis stage revealed two genes with DNA transposon Castaway in the 5′-flanking region. Biosci Biotechnol Biochem 68:1315–1323
Yamaguchi-Shinozaki K, Shinozaki K (1994) A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell 6:251–264
Yi SY, Hwang BK (1998) Molecular cloning and characterization of a new basic peroxidase cDNA from soybean hypocotyls infected with Phytophthora sojae f. sp. glycines. Mol Cells 8:556–564
Yi N, Kim YS, Jeong MH, Oh SJ, Jeong JS, Park SH, Jung H, Choi YD, Kim JK (2010) Functional analysis of six drought-inducible promoters in transgenic rice plants throughout all stages of plant growth. Planta 232:743–754
Zhu JK (2001) Cell signaling under salt, water and cold stresses. Curr Opin Plant Biol 4:401–406
Acknowledgments
This work was supported by a grant from the Next-Generation BioGreen 21 Program (Plant Molecular Breeding Center No. PJ008197), Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kim, SH., Choi, HS., Cho, YC. et al. Cold-Responsive Regulation of a Flower-Preferential Class III Peroxidase Gene, OsPOX1, in Rice (Oryza sativa L.). J. Plant Biol. 55, 123–131 (2012). https://doi.org/10.1007/s12374-011-9194-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12374-011-9194-3