Abstract
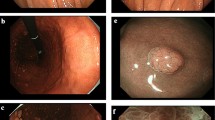
A 68-year-old man with no symptoms presented to Hokkaido University Hospital for esophagogastroduodenoscopy screening. He had a history of Helicobacter pylori eradication. Initial esophagogastroduodenoscopy showed no gastric cobblestone-like mucosa or gastric cracked mucosa. After 1 year, he received esomeprazole (20 mg) once daily for heartburn at another hospital. Esophagogastroduodenoscopy was performed after 2 years of esomeprazole administration. Endoscopic findings showed that after H. pylori eradication, according to the Kyoto classification, gastric cobblestone-like mucosa presented in the gastric body area. Dilation of the oval crypt opening and intervening part in the gastric cobblestone-like mucosa was detected by endoscopy with narrow band imaging. Endoscopic ultrasonography revealed a thick gastric second layer and sporadic small a-echoic lesions in the low-echoic thickened second layer in the gastric cobblestone-like mucosa. The gastric cobblestone-like mucosa biopsy specimen showed parietal cell protrusions and oxyntic gland dilatations. Recently, we reported that gastric mucosal changes such as gastric cracked mucosa and gastric cobblestone-like mucosa were caused by proton-pump inhibitors; however, the gastric cobblestone-like mucosa was not examined by endoscopic ultrasonography. In this case, endoscopic ultrasonography findings suggested that oxyntic gland dilatations caused the elevated gastric mucosa, such as gastric cobblestone-like mucosa, from the use of proton-pump inhibitors.




Similar content being viewed by others
References
Haastrup PF, Paulsen MS, Christensen RD, et al. Medical and non-medical predictors of initiating long-term use of proton pump inhibitors: a nationwide cohort study of first-time users during a 10-year period. Aliment Pharmacol Ther. 2016;44:78–87.
Boutet R, Wilcock M, MacKenzie I. Survey on repeat prescribing for acid suppression drugs in primary care in Cornwall and the Isles of Scilly. Aliment Pharmacol Ther. 1999;13:813–7.
Leonard J, Marshall JK, Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol. 2007;102:2047–56.
Corley DA, Kubo A, Zhao W, et al. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology. 2010;139:93–101.
Hongo M, Fujimoto K, Gastric Polyps Study G. Incidence and risk factor of fundic gland polyp and hyperplastic polyp in long-term proton pump inhibitor therapy: a prospective study in Japan. J Gastroenterol. 2010;45:618–24.
Jalving M, Koornstra JJ, Wesseling J, et al. Increased risk of fundic gland polyps during long-term proton pump inhibitor therapy. Aliment Pharmacol Ther. 2006;24:1341–8.
Hatano Y, Haruma K, Ayaki M, et al. Black spot, a novel gastric finding potentially induced by proton pump inhibitors. Intern Med. 2016;55:3079–84.
Kato M. Endoscopic findings of H. pylori infection. In: Suzuki H, Warren R, Marshall B, editors. Helicobacter pylori. Japan: Springer; 2016. p. 157–67.
Kumar KR, Iqbal R, Coss E, et al. Helicobacter gastritis induces changes in the oxyntic mucosa indistinguishable from the effects of proton pump inhibitors. Hum Pathol. 2013;44:2706–10.
Stolte M, Bethke B, Ruhl G, et al. Omeprazole-induced pseudohypertrophy of gastric parietal cells. Z Gastroenterol. 1992;30:134–8.
Miyamoto S, Kato M, Tsuda M, et al. Gastric mucosal cracked and cobblestone-like changes by the use of proton pump inhibitors. Dig Endosc. 2016. doi:10.1111/den.12765
Naruki S, Fujino T, Ohnuma S, et al. Histopathologic and immunohistochemical characterization of human gastric oxyntic mucosa with parietal cell protrusions and investigation into the association between such mucosal changes of the stomach and use of proton pump inhibitors. J St Marianna Univ. 2015;6:119–30.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest:
Mototsugu Kato has received honoraria from Eisai Co., Ltd.; Daiichi Sankyo Co., Ltd.; and AstraZeneca K.K. and has received scholarship grants from Eisai Co., Ltd.; Takeda Pharmaceutical Co., Ltd.; Daiichi Sankyo Co., Ltd.; and AstraZeneca K.K. and Astellas Pharma Inc. Y. Shoko Ono has been involved in speaking and teaching commitments for Eisai Co., Ltd.; Daiichi Sankyo Co.; and AstraZeneca K.K. Yuichi Shimizu has received research grants from Takeda Pharmaceutical Co.; Eisai Co., Ltd.; and AstraZeneca K.K. Naoya Sakamoto has received honoraria from Bristol-Myers Squibb and Janssen Pharmaceutical K.K. and research funding from Chugai Pharmaceutical Co., Ltd. and Bristol-Myers Squibb. Naoya Sakamoto received honoraria from Bristol-Myers Squibb and Janssen Pharmaceutical K.K. and research funding from Chugai Pharmaceutical Co., Ltd. and Bristol-Myers Squibb.
Human Rights:
All procedures followed have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed Consent:
Informed consent was obtained from this patient for being included in the study.
Rights and permissions
About this article
Cite this article
Miyamoto, S., Kudo, T., Kato, M. et al. Endoscopic ultrasonography features of gastric mucosal cobblestone-like changes from a proton-pump inhibitor. Clin J Gastroenterol 10, 220–223 (2017). https://doi.org/10.1007/s12328-017-0724-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-017-0724-5